Long-Range Control of Renin Gene Expression in Tsukuba Hypertensive Mice
- PMID: 27861631
- PMCID: PMC5115840
- DOI: 10.1371/journal.pone.0166974
Long-Range Control of Renin Gene Expression in Tsukuba Hypertensive Mice
Abstract
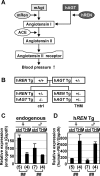
Renin, a rate-limiting enzyme in the renin-angiotensin system, is regulated to maintain blood pressure homeostasis: renin gene expression in the kidney is suppressed in a hypertensive environment. We found that expression of a 15-kb human RENIN (hREN) transgene was aberrantly upregulated (>4.2-fold), while the endogenous mouse renin (mRen) gene was suppressed (>1.7-fold) in Tsukuba hypertensive mice (THM), a model for genetically induced hypertension. We then generated transgenic mice using a 13-kb mRen gene fragment that was homologous to the 15-kb hREN transgene and found that its expression was also upregulated (>3.1-fold) in THM, suggesting that putative silencing elements of the renin genes were distally located in the loci. We next examined the possible role of a previously identified mouse distal enhancer (mdE) located outside of the 13-kb mRen gene fragment. Deletion of the mdE in the context of a 156-kb mRen transgene did not affect its transcriptional repression in THM, implying that although the silencing element of the mRen gene is located within the 156-kb fragment tested, it is distinct from the mdE. Consistent with these results, deletion of the 63-kb region upstream of the mdE from the endogenous mRen gene locus abrogated its transcriptional repression in THM. We finally tested whether dysregulation of the short renin transgenes also occurred in the fetal or neonatal kidneys of THM and found that their expression was not aberrantly upregulated, demonstrating that aberrant regulation of short renin transgenes commences sometime between neonate and adult periods.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






Similar articles
-
Homeostatic Response of Mouse renin Gene Transcription in a Hypertensive Environment Is Mediated by a Novel 5' Enhancer.Mol Cell Biol. 2018 Mar 15;38(7):e00566-17. doi: 10.1128/MCB.00566-17. Print 2018 Apr 1. Mol Cell Biol. 2018. PMID: 29358217 Free PMC article.
-
Absence of peroxisome proliferator-activated receptor-alpha abolishes hypertension and attenuates atherosclerosis in the Tsukuba hypertensive mouse.Hypertension. 2007 Nov;50(5):945-51. doi: 10.1161/HYPERTENSIONAHA.107.094268. Epub 2007 Oct 1. Hypertension. 2007. PMID: 17909121
-
Reversal of the suppressed kidney renin level in the hypertensive transgenic rat TGR(mRen-2)27 by angiotensin converting enzyme inhibition.Am J Hypertens. 1995 Oct;8(10 Pt 1):1031-9. doi: 10.1016/0895-7061(95)00270-7. Am J Hypertens. 1995. PMID: 8845072
-
Transcriptional regulation of renin: an update.Hypertension. 2005 Jan;45(1):3-8. doi: 10.1161/01.HYP.0000149717.55920.45. Epub 2004 Nov 15. Hypertension. 2005. PMID: 15545507 Review.
-
Structure, expression, and regulation of the murine renin genes.Hypertension. 1991 Oct;18(4):446-57. doi: 10.1161/01.hyp.18.4.446. Hypertension. 1991. PMID: 1916990 Review.
Cited by
-
Homeostatic Response of Mouse renin Gene Transcription in a Hypertensive Environment Is Mediated by a Novel 5' Enhancer.Mol Cell Biol. 2018 Mar 15;38(7):e00566-17. doi: 10.1128/MCB.00566-17. Print 2018 Apr 1. Mol Cell Biol. 2018. PMID: 29358217 Free PMC article.
References
-
- Bader M, Ganten D. Regulation of renin: new evidence from cultured cells and genetically modified mice. J Mol Med (Berl). 2000;78(3):130–9. Epub 2000/06/27. . - PubMed
-
- Tanimoto K, Sugiyama F, Goto Y, Ishida J, Takimoto E, Yagami K, et al. Angiotensinogen-deficient mice with hypotension. J Biol Chem. 1994;269(50):31334–7. Epub 1994/12/16. . - PubMed
-
- Thompson MW, Smith SB, Sigmund CD. Regulation of human renin mRNA expression and protein release in transgenic mice. Hypertension. 1996;28(2):290–6. Epub 1996/08/01. . - PubMed
-
- Keen HL, Sigmund CD. Paradoxical regulation of short promoter human renin transgene by angiotensin ii. Hypertension. 2001;37(2 Pt 2):403–7. Epub 2001/03/07. . - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

