Pdcd2l Promotes Palmitate-Induced Pancreatic Beta-Cell Apoptosis as a FoxO1 Target Gene
- PMID: 27861641
- PMCID: PMC5115776
- DOI: 10.1371/journal.pone.0166692
Pdcd2l Promotes Palmitate-Induced Pancreatic Beta-Cell Apoptosis as a FoxO1 Target Gene
Abstract
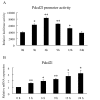
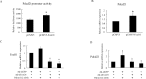
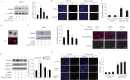
Transcription factor FoxO1 is a key regulator of the insulin-signaling pathway, and is reported to play important roles in pancreatic β cell differentiation, proliferation, apoptosis and stress resistance. The multifunctional nature of FoxO1 is due to its regulation of various downstream targets. Previous studies in our lab identified potential FoxO1 target genes using the ChIP-DSL technique and one of those genes, Pdcd2l, was selected for further study. We found that the expression of Pdcd2l was increased with palmitate treatment; the luciferase assay result revealed that enhanced Pdcd2l promoter activity was responsible for the elevation of Pdcd2l expression. ChIP-PCR was performed to confirm the combination of FoxO1 to Pdcd2l promoter, result showing that FoxO1 could bind to Pdcd2l promoter and this binding was further enhanced after palmitate treatment. Overexpression of FoxO1 significantly induced Pdcd2l promoter activity, leading to increased mRNA level; consistently, interference of FoxO1 abolished the increment of Pdcd2l gene expression triggered by palmitate treatment. In addition, overexpression of Pdcd2l could further increase the percentage of apoptotic cells induced by palmitate incubation, whilst interference of Pdcd2l partially reversed the palmitate-induced apoptosis together with activated Caspase-3, indicating that the latter may play a part in this process. Therefore, in this study, we confirmed the binding of FoxO1 to the Pdcd2l gene promoter and studied the role of Pdcd2l in β cells for the first time. Our results suggested that FoxO1 may exert its activity partially through the regulation of Pdcd2l in palmitate-induced β cell apoptosis and could help to clarify the molecular mechanisms of β cell failure in type 2 diabetes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Identification of direct forkhead box O1 targets involved in palmitate-induced apoptosis in clonal insulin-secreting cells using chromatin immunoprecipitation coupled to DNA selection and ligation.Diabetologia. 2012 Oct;55(10):2703-2712. doi: 10.1007/s00125-012-2643-9. Epub 2012 Jul 20. Diabetologia. 2012. PMID: 22810813
-
Curcumin attenuates palmitate-induced apoptosis in MIN6 pancreatic β-cells through PI3K/Akt/FoxO1 and mitochondrial survival pathways.Apoptosis. 2015 Nov;20(11):1420-32. doi: 10.1007/s10495-015-1150-0. Apoptosis. 2015. PMID: 26330141
-
Forkhead box O1 mediates defects in palmitate-induced insulin granule exocytosis by downregulation of calcium/calmodulin-dependent serine protein kinase expression in INS-1 cells.Diabetologia. 2015 Jun;58(6):1272-81. doi: 10.1007/s00125-015-3561-4. Epub 2015 Mar 22. Diabetologia. 2015. PMID: 25796372
-
The multifaceted function of FoxO1 in pancreatic β-cell dysfunction and insulin resistance: Therapeutic potential for type 2 diabetes.Life Sci. 2025 Mar 1;364:123384. doi: 10.1016/j.lfs.2025.123384. Epub 2025 Jan 10. Life Sci. 2025. PMID: 39798646 Review.
-
The Essential Role of FoxO1 in the Regulation of Macrophage Function.Biomed Res Int. 2022 Aug 11;2022:1068962. doi: 10.1155/2022/1068962. eCollection 2022. Biomed Res Int. 2022. PMID: 35993049 Free PMC article. Review.
Cited by
-
Influences of sterol regulatory element binding protein-1c silencing on glucose production in HepG2 cells treated with free fatty acid.Lipids Health Dis. 2019 Apr 6;18(1):89. doi: 10.1186/s12944-019-1026-3. Lipids Health Dis. 2019. PMID: 30954075 Free PMC article.
-
Proteomics reveals that nanoplastics with different sizes induce hepatocyte apoptosis in mice through distinct mechanisms involving mitophagy dysregulation and cell cycle arrest.Toxicol Res (Camb). 2024 Nov 12;13(6):tfae188. doi: 10.1093/toxres/tfae188. eCollection 2024 Dec. Toxicol Res (Camb). 2024. PMID: 39539253
-
GRP75 mediates endoplasmic reticulum-mitochondria coupling during palmitate-induced pancreatic β-cell apoptosis.J Biol Chem. 2021 Dec;297(6):101368. doi: 10.1016/j.jbc.2021.101368. Epub 2021 Oct 29. J Biol Chem. 2021. PMID: 34756890 Free PMC article.
-
microRNA-802 is involved in palmitate-induced damage to pancreatic β cells through repression of sirtuin 6.Int J Clin Exp Pathol. 2017 Nov 1;10(11):11300-11307. eCollection 2017. Int J Clin Exp Pathol. 2017. PMID: 31966484 Free PMC article.
-
Drosophila Trus, the orthologue of mammalian PDCD2L, is required for proper cell proliferation, larval developmental timing, and oogenesis.bioRxiv [Preprint]. 2024 Oct 26:2024.10.24.620039. doi: 10.1101/2024.10.24.620039. bioRxiv. 2024. Update in: PLoS Genet. 2025 Jun 27;21(6):e1011469. doi: 10.1371/journal.pgen.1011469. PMID: 39484569 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

