High Smad7 sustains inflammatory cytokine response in refractory coeliac disease
- PMID: 27861825
- PMCID: PMC5290231
- DOI: 10.1111/imm.12690
High Smad7 sustains inflammatory cytokine response in refractory coeliac disease
Abstract
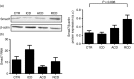
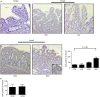
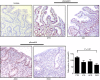
Refractory coeliac disease (RCD) is a form of coeliac disease (CD) resistant to gluten-free diet and associated with elevated risk of complications. Many effector cytokines over-produced in the gut of patients with RCD are supposed to amplify the tissue-destructive immune response, but it remains unclear if the RCD-associated mucosal inflammation is sustained by defects in counter-regulatory mechanisms. The aim of the present study was to determine whether RCD-related inflammation is marked by high Smad7, an intracellular inhibitor of transforming growth factor-β1 (TGF-β1 ) activity. Smad7 was evaluated in duodenal biopsy samples of patients with RCD, patients with active CD, patients with inactive CD and healthy controls by Western blotting, immunohistochemistry and real-time PCR. In the same samples, TGF-β1 and phosphorylated (p)-Smad2/3 were evaluated by ELISA and immunohistochemistry, respectively. Pro-inflammatory cytokine expression was evaluated in RCD samples cultured with Smad7 sense or antisense oligonucleotide. Smad7 protein, but not RNA, expression was increased in RCD compared with active and inactive CD patients and healthy controls and this was associated with defective TGF-β1 signalling, as marked by diminished p-Smad2/3 expression. TGF-β1 protein content did not differ among groups. Knockdown of Smad7 in RCD biopsy samples reduced interleukin-6 and tumour necrosis factor-α expression. In conclusion, in RCD, high Smad7 associates with defective TGF-β1 signalling and sustains inflammatory cytokine production. These results indicate a novel mechanism by which the mucosal cytokine response is amplified in RCD and suggest that targeting Smad7 can be therapeutically useful in RCD.
Keywords: gluten; inflammation; mucosal immune response; transforming growth factor-β.
© 2016 John Wiley & Sons Ltd.
Figures

References
-
- Daum S, Cellier C, Mulder CJ. Refractory coeliac disease. Best Pract Res Clin Gastroenterol 2005; 19:413–24. - PubMed
-
- Abdulkarim AS, Burgart LJ, See J, Murray JA. Etiology of nonresponsive celiac disease: results of a systematic approach. Am J Gastroenterol 2002; 97:2016–21. - PubMed
-
- Al‐Toma A, Verbeek WH, Mulder CJ. Update on the management of refractory coeliac disease. J Gastrointestin Liver Dis 2007; 16:57–63. - PubMed
-
- Leffler DA, Dennis M, Hyett B, Kelly E, Schuppan D, Kelly CP et al Etiologies and predictors of diagnosis in nonresponsive celiac disease. Clin Gastroenterol Hepatol 2007; 5:445–50. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

