Potassium channels in the heart: structure, function and regulation
- PMID: 27861921
- PMCID: PMC5374109
- DOI: 10.1113/JP272864
Potassium channels in the heart: structure, function and regulation
Abstract
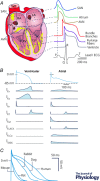
This paper is the outcome of the fourth UC Davis Systems Approach to Understanding Cardiac Excitation-Contraction Coupling and Arrhythmias Symposium, a biannual event that aims to bring together leading experts in subfields of cardiovascular biomedicine to focus on topics of importance to the field. The theme of the 2016 symposium was 'K+ Channels and Regulation'. Experts in the field contributed their experimental and mathematical modelling perspectives and discussed emerging questions, controversies and challenges on the topic of cardiac K+ channels. This paper summarizes the topics of formal presentations and informal discussions from the symposium on the structural basis of voltage-gated K+ channel function, as well as the mechanisms involved in regulation of K+ channel gating, expression and membrane localization. Given the critical role for K+ channels in determining the rate of cardiac repolarization, it is hardly surprising that essentially every aspect of K+ channel function is exquisitely regulated in cardiac myocytes. This regulation is complex and highly interrelated to other aspects of myocardial function. K+ channel regulatory mechanisms alter, and are altered by, physiological challenges, pathophysiological conditions, and pharmacological agents. An accompanying paper focuses on the integrative role of K+ channels in cardiac electrophysiology, i.e. how K+ currents shape the cardiac action potential, and how their dysfunction can lead to arrhythmias, and discusses K+ channel-based therapeutics. A fundamental understanding of K+ channel regulatory mechanisms and disease processes is fundamental to reveal new targets for human therapy.
Keywords: gating; heterogeneity; phosphorylation; trafficking.
© 2016 The Authors. The Journal of Physiology © 2016 The Physiological Society.
Figures


Comment in
-
Size matters, proportion too: coupling of experiments and theory reveals relative roles of K+ channels in action potential stability.J Physiol. 2017 Apr 1;595(7):2319-2320. doi: 10.1113/JP273665. Epub 2017 Jan 11. J Physiol. 2017. PMID: 28004403 Free PMC article. No abstract available.
References
-
- Aguilar M & Nattel S (2015). The past, present, and potential future of sodium channel block as an atrial fibrillation suppressing strategy. J Cardiovasc Pharmacol 66, 432–440. - PubMed
-
- Aimond F, Kwak SP, Rhodes KJ & Nerbonne JM (2005). Accessory Kvβ1 subunits differentially modulate the functional expression of voltage‐gated K+ channels in mouse ventricular myocytes. Circ Res 96, 451–458. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL081562/HL/NHLBI NIH HHS/United States
- R01 HL085727/HL/NHLBI NIH HHS/United States
- R01 HL122421/HL/NHLBI NIH HHS/United States
- T32 HL007822/HL/NHLBI NIH HHS/United States
- R01 HL123526/HL/NHLBI NIH HHS/United States
- I01 CX001490/CX/CSRD VA/United States
- R01 HL088427/HL/NHLBI NIH HHS/United States
- R01 HL131517/HL/NHLBI NIH HHS/United States
- R01 HL030077/HL/NHLBI NIH HHS/United States
- R01 HL121059/HL/NHLBI NIH HHS/United States
- R01 HL137228/HL/NHLBI NIH HHS/United States
- R01 HL098200/HL/NHLBI NIH HHS/United States
- I01 BX000576/BX/BLRD VA/United States
- R01 HL085844/HL/NHLBI NIH HHS/United States
- R13 HL132515/HL/NHLBI NIH HHS/United States
- R01 HL105242/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

