Allogeneic major histocompatibility complex-mismatched equine bone marrow-derived mesenchymal stem cells are targeted for death by cytotoxic anti-major histocompatibility complex antibodies
- PMID: 27862236
- PMCID: PMC5425313
- DOI: 10.1111/evj.12647
Allogeneic major histocompatibility complex-mismatched equine bone marrow-derived mesenchymal stem cells are targeted for death by cytotoxic anti-major histocompatibility complex antibodies
Abstract
Background: Allogeneic mesenchymal stem cells (MSCs) are a promising cell source for treating musculoskeletal injuries in horses. Controversy exists, however, over whether major histocompatibility complex (MHC)-mismatched MSCs are recognised by the recipient immune system and targeted for death by a cytotoxic antibody response.
Objectives: To determine if cytotoxic anti-MHC antibodies generated in vivo following MHC-mismatched MSC injections are capable of initiating complement-dependent cytotoxicity of MSCs.
Study design: Experimental controlled study.
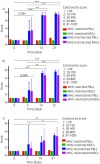
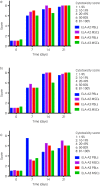
Methods: Antisera previously collected at Days 0, 7, 14 and 21 post-injection from 4 horses injected with donor MHC-mismatched equine leucocyte antigen (ELA)-A2 haplotype MSCs and one control horse injected with donor MHC-matched ELA-A2 MSCs were utilised in this study. Antisera were incubated with ELA-A2 MSCs before adding complement in microcytotoxicity assays and cell death was analysed via eosin dye exclusion. ELA-A2 peripheral blood leucocytes (PBLs) were used in the assays as a positive control.
Results: Antisera from all 4 horses injected with MHC-mismatched MSCs contained antibodies that caused the death of ELA-A2 haplotype MSCs in the microcytotoxicity assays. In 2 of the 4 horses, antibodies were present as early as Day 7 post-injection. MSC death was consistently equivalent to that of ELA-A2 haplotype PBL death at all time points and antisera dilutions. Antisera from the control horse that was injected with MHC-matched MSCs did not contain cytotoxic ELA-A2 antibodies at any of the time points examined.
Main limitations: This study examined MSC death in vitro only and utilized antisera from a small number of horses.
Conclusions: The cytotoxic antibody response induced in recipient horses following injection with donor MHC-mismatched MSCs is capable of killing donor MSCs in vitro. These results suggest that the use of allogeneic MHC-mismatched MSCs must be cautioned against, not only for potential adverse events, but also for reduced therapeutic efficacy due to targeted MSC death.
Keywords: allogeneic; antibody response; complement-dependent cytotoxicity; horse; major histocompatibility complex-mismatched; mesenchymal stem cell.
© 2016 The Authors Equine Veterinary Journal published by John Wiley & Sons Ltd on behalf of EVJ Ltd.
Figures

References
-
- Schnabel, L.V. , Lynch, M.E. , van der Meulen, M.C. , Yeager, A.E. , Kornatowski, M.A. and Nixon, A.J. (2009) Mesenchymal stem cells and insulin‐like growth factor‐I gene‐enhanced mesenchymal stem cells improve structural aspects of healing in equine flexor digitorum superficialis tendons. J. Orthop. Res. 27, 1392–1398. - PubMed
-
- Crovace, A. , Lacitignola, L. , Rossi, G. and Francioso, E. (2010) Histological and immunohistochemical evaluation of autologous cultured bone marrow mesenchymal stem cells and bone marrow mononucleated cells in collagenase‐induced tendinitis of equine superficial digital flexor tendon. Vet. Med. Int. 2010, 250978. - PMC - PubMed
-
- Conze, P. , van Schie, H.T. , van Weeren, R. , Staszyk, C. , Conrad, S. , Skutella, T. , Hopster, K. , Rohn, K. , Stadler, P. and Geburek, F. (2014) Effect of autologous adipose tissue‐derived mesenchymal stem cells on neovascularization of artificial equine tendon lesions. Regen. Med. 9, 743–757. - PubMed
-
- Ferris, D.J. , Frisbie, D.D. , Kisiday, J.D. , McIlwraith, C.W. , Hague, B.A. , Major, M.D. , Schneider, R.K. , Zubrod, C.J. , Kawcak, C.E. and Goodrich, L.R. (2014) Clinical outcome after intra‐articular administration of bone marrow derived mesenchymal stem cells in 33 horses with stifle injury. Vet. Surg. 43, 255–265. - PubMed
-
- Pacini, S. , Spinabella, S. , Trombi, L. , Fazzi, R. , Galimberti, S. , Dini, F. , Carlucci, F. and Petrini, M. (2007) Suspension of bone marrow‐derived undifferentiated mesenchymal stromal cells for repair of superficial digital flexor tendon in race horses. Tissue Eng. 13, 2949–2955. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

