Chirality Transfer in Gold(I)-Catalysed Hydroalkoxylation of 1,3-Disubstituted Allenes
- PMID: 27862422
- PMCID: PMC5215423
- DOI: 10.1002/chem.201603918
Chirality Transfer in Gold(I)-Catalysed Hydroalkoxylation of 1,3-Disubstituted Allenes
Abstract
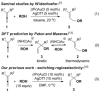
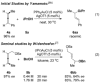
Gold(I)-catalysed intermolecular hydroalkoxylation of enantioenriched 1,3-disubstituted allenes was previously reported to occur with poor chirality transfer due to rapid allene racemisation. The first intermolecular hydroalkoxylation of allenes with efficient chirality transfer is reported here, exploiting conditions that suppress allene racemisation. A full substrate scope study reveals that excellent regio- and stereoselectivities are achieved when a σ-withdrawing substituent is present.
Keywords: alcohols; allenes; chirality transfer; ether; gold.
© 2016 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Figures







Similar articles
-
Gold(I)-Catalysed Hydroarylation of 1,3-Disubstituted Allenes with Efficient Axial-to-Point Chirality Transfer.Chemistry. 2018 May 11;24(27):7002-7009. doi: 10.1002/chem.201800209. Epub 2018 Apr 26. Chemistry. 2018. PMID: 29542832
-
Regio- and stereoselective synthesis of alkyl allylic ethers via gold(I)-catalyzed intermolecular hydroalkoxylation of allenes with alcohols.Org Lett. 2008 May 15;10(10):2079-81. doi: 10.1021/ol800646h. Epub 2008 Apr 16. Org Lett. 2008. PMID: 18412355
-
Chirality Transfer in Gold(I)-Catalysed Direct Allylic Etherifications of Unactivated Alcohols: Experimental and Computational Study.Chemistry. 2015 Sep 21;21(39):13748-57. doi: 10.1002/chem.201501607. Epub 2015 Aug 6. Chemistry. 2015. PMID: 26248980 Free PMC article.
-
Gold(I)-catalyzed intermolecular hydroalkoxylation of allenes: a DFT study.Org Lett. 2009 Jun 4;11(11):2237-40. doi: 10.1021/ol9004646. Org Lett. 2009. PMID: 19400580
-
Unveiling the Chemistry and Synthetic Potential of Catalytic Cycloaddition Reaction of Allenes: A Review.Molecules. 2023 Jan 10;28(2):704. doi: 10.3390/molecules28020704. Molecules. 2023. PMID: 36677762 Free PMC article. Review.
Cited by
-
Zinc Iodide Catalyzed Synthesis of Trisubstituted Allenes from Terminal Alkynes and Ketones.ACS Omega. 2021 Aug 31;6(36):23329-23346. doi: 10.1021/acsomega.1c03092. eCollection 2021 Sep 14. ACS Omega. 2021. PMID: 34549133 Free PMC article.
-
Rapid Iododeboronation with and without Gold Catalysis: Application to Radiolabelling of Arenes.Chemistry. 2018 Jan 19;24(4):937-943. doi: 10.1002/chem.201704534. Epub 2017 Dec 14. Chemistry. 2018. PMID: 29105856 Free PMC article.
References
-
- For selected reviews on gold catalysis, see:
-
- Fürstner A., Davies P. W., Angew. Chem. Int. Ed. 2007, 46, 3410–3449; - PubMed
- Angew. Chem. 2007, 119, 3478–3519;
-
- Gorin D. J., Toste F. D., Nature 2007, 446, 395–403; - PubMed
-
- Hashmi A. S. K., Hutchings G. J., Angew. Chem. Int. Ed. 2006, 45, 7896–7936; - PubMed
- Angew. Chem. 2006, 118, 8064–8105;
-
- Hashmi A. S. K., Chem. Rev. 2007, 107, 3180–3211; - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

