Randomized assessment of imatinib in patients with acute ischaemic stroke treated with intravenous thrombolysis
- PMID: 27862464
- PMCID: PMC5573589
- DOI: 10.1111/joim.12576
Randomized assessment of imatinib in patients with acute ischaemic stroke treated with intravenous thrombolysis
Abstract
Background: Imatinib, a tyrosine kinase inhibitor, has been shown to restore blood-brain barrier integrity and reduce infarct size, haemorrhagic transformation and cerebral oedema in stroke models treated with tissue plasminogen activator. We evaluated the safety of imatinib, based on clinical and neuroradiological data, and its potential influence on neurological and functional outcomes.
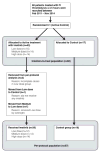
Methods: A phase II randomized trial was performed in patients with acute ischaemic stroke treated with intravenous thrombolysis. A total of 60 patients were randomly assigned to four groups [3 (active): 1 (control)]; the active treatment groups received oral imatinib for 6 days at three dose levels (400, 600 and 800 mg). Primary outcome was any adverse event; secondary outcomes were haemorrhagic transformation, cerebral oedema, neurological severity on the National Institutes of Health Stroke Scale (NIHSS) at 7 days and at 3 months and functional outcomes on the modified Rankin scale (mRS).
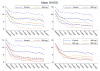
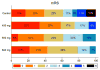
Results: Four serious adverse events were reported, which resulted in three deaths (one in the control group and two in the 400-mg dose group; one patient in the latter group did not receive active treatment and the other received two doses). Nonserious adverse events were mostly mild, resulting in full recovery. Imatinib ameliorated neurological outcomes with an improvement of 0.6 NIHSS points per 100 mg imatinib (P = 0.02). For the 800-mg group, the mean unadjusted and adjusted NIHSS improvements were 4 (P = 0.037) and 5 points (P = 0.012), respectively, versus controls. Functional independence (mRS 0-2) increased by 18% versus controls (61 vs. 79; P = 0.296).
Conclusion: This phase II study showed that imatinib is safe and tolerable and may reduce neurological disability in patients treated with intravenous thrombolysis after ischaemic stroke. A confirmatory randomized trial is currently underway.
Keywords: cerebral infarct; cerebral oedema; imatinib; intracerebral haemorrhage; stroke; thrombolysis.
© 2016 The Authors. Journal of Internal Medicine published by John Wiley & Sons Ltd on behalf of Association for Publication of The Journal of Internal Medicine.
Conflict of interest statement
Nils Wahlgren and Ulf Eriksson have applied for a patent for the use of imatinib in acute stroke.
All other authors have no conflicts of interest.
Details regarding study coordination, contribution to the manuscript, members of the safety committee, study investigators and the research nurses responsible for patient recruitment are available as web-material (W7).
Figures
References
-
- Abbruscato TJ, Davis TP. Combination of hypoxia/aglycemia compromises in vitro blood-brain barrier integrity. J Pharmacol ExpTher. 1999;289:668–675. - PubMed
-
- Fischer S, Clauss M, Wiesnet M, Renz D, Schaper W, Karliczek GF. Hypoxia induces permeability in brain microvessel endothelial cells via VEGF and NO. Am J Physiol. 1999;276:C812–820. - PubMed
-
- Latour LL, Kang DW, Ezzeddine MA, Chalela JA, Warach S. Early blood-brain disruption in human focal brain ischemia. Ann Neurol. 2004;56:468–477. - PubMed
-
- Sandoval KE, Witt KA. Blood-brain barrier tight junction permeability and ischaemic stroke. Neurobiology of Disease. 2008;32:200–219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

