Role of ammonium in the ionization of phosphatidylcholines during electrospray mass spectrometry
- PMID: 27862466
- PMCID: PMC5253255
- DOI: 10.1002/rcm.7788
Role of ammonium in the ionization of phosphatidylcholines during electrospray mass spectrometry
Abstract
Rationale: Electrospray mass spectrometry methods for the analysis of phosphatidylcholines (PCs) routinely include ammonium acetate or ammonium formate in the mobile phase. In an effort to justify and optimize the use of these additives, we investigated possible functions of ammonium compounds in the ionization of PCs.
Methods: Because PCs contain a quaternary amine, the role of ammonium in neutralizing the negatively charged phosphate group was investigated by using deuterated ammonium acetate, adjusting the pH, varying the organic solvent composition, and by comparing the additives ammonium acetate, ammonium formate and ammonium bicarbonate. Seven PC standards were measured ranging from lyso 1-palmitoyl-sn-glycero-3-phosphocholine to 1,2-dieicosapentaenoyl-sn-glycero-3-phosphocholine as well as a mixture of PCs in a krill oil dietary supplement.
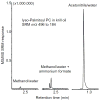
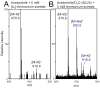
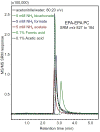
Results: Under all conditions tested, aqueous acetonitrile provided more abundant formation of protonated PCs than did aqueous methanol. Regardless of the mobile phase composition and electrospray ion source parameters, no [M + NH4 ]+ ions were detected. Adding deuterated ammonium acetate to the mobile phase failed to form deuterated PCs, indicating that ammonium is not the source of the proton that neutralizes the phosphate negative charge. Instead, water was the source of the proton as deuterated water resulted in the formation of [M + D]+ ions. Addition of organic acids, ammonium formate, ammonium acetate, or ammonium bicarbonate to the mobile phase did not enhance and in most cases suppressed PC ionization.
Conclusions: Ammonium compounds and organic acids can suppress ionization of PCs when using an aqueous acetonitrile mobile phase during electrospray. Copyright © 2016 John Wiley & Sons, Ltd.
Copyright © 2016 John Wiley & Sons, Ltd.
Figures

Similar articles
-
Identification and quantification of phosphatidylcholines containing very-long-chain polyunsaturated fatty acid in bovine and human retina using liquid chromatography/tandem mass spectrometry.J Chromatogr A. 2010 Dec 3;1217(49):7738-48. doi: 10.1016/j.chroma.2010.10.039. Epub 2010 Oct 14. J Chromatogr A. 2010. PMID: 21035124
-
Simple LC-MS Method for Differentiation of Isobaric Phosphatidylserines and Phosphatidylcholines with Deuterated Mobile Phase Additives.Anal Chem. 2016 Sep 20;88(18):9103-10. doi: 10.1021/acs.analchem.6b02063. Epub 2016 Aug 26. Anal Chem. 2016. PMID: 27532481 Free PMC article.
-
Analysis of metal complex azo dyes by high-performance liquid chromatography/electrospray ionization mass spectrometry and multistage mass spectrometry.Rapid Commun Mass Spectrom. 2000;14(20):1881-8. doi: 10.1002/1097-0231(20001030)14:20<1881::AID-RCM107>3.0.CO;2-I. Rapid Commun Mass Spectrom. 2000. PMID: 11013416
-
Forced degradation and impurity profiling: recent trends in analytical perspectives.J Pharm Biomed Anal. 2013 Dec;86:11-35. doi: 10.1016/j.jpba.2013.07.013. Epub 2013 Jul 31. J Pharm Biomed Anal. 2013. PMID: 23969330 Review.
-
Sensitivity enhancement in liquid chromatography/atmospheric pressure ionization mass spectrometry using derivatization and mobile phase additives.J Chromatogr B Analyt Technol Biomed Life Sci. 2005 Oct 25;825(2):98-110. doi: 10.1016/j.jchromb.2005.04.021. J Chromatogr B Analyt Technol Biomed Life Sci. 2005. PMID: 15897015 Review.
Cited by
-
Combined Treatment with Sodium-Glucose Cotransporter-2 Inhibitor (Canagliflozin) and Dipeptidyl Peptidase-4 Inhibitor (Teneligliptin) Alleviates NASH Progression in A Non-Diabetic Rat Model of Steatohepatitis.Int J Mol Sci. 2020 Mar 21;21(6):2164. doi: 10.3390/ijms21062164. Int J Mol Sci. 2020. PMID: 32245205 Free PMC article.
-
Dynamic mass spectrometry probe for electrospray ionization mass spectrometry monitoring of bioreactors for therapeutic cell manufacturing.Biotechnol Bioeng. 2019 Jan;116(1):121-131. doi: 10.1002/bit.26832. Epub 2018 Nov 6. Biotechnol Bioeng. 2019. PMID: 30199089 Free PMC article.
References
-
- Canty DJ, Zeisel SH. Lecithin and choline in human health and disease. Nutr Rev. 1994;52:327. - PubMed
-
- Kawashima Y, Mizuguchi H, Kozuka H. Modulation by dietary oils and clofibric acid of arachidonic acid content in phosphatidylcholine in liver and kidney of rat: Effects on prostaglandin formation in kidney. Biochim Biophys Acta. 1994;1210:187. - PubMed
-
- Exton JH. Signaling through phosphatidylcholine breakdown. J Biol Chem. 1990;265:1. - PubMed
-
- Kim H-Y, Wang T-CL, Ma Y-C. Liquid chromatography/mass spectrometry of phospholipids using electrospray ionization. Anal Chem. 1994;66:3977. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
