All-trans retinoic acid preconditioning enhances proliferation, angiogenesis and migration of mesenchymal stem cell in vitro and enhances wound repair in vivo
- PMID: 27862498
- PMCID: PMC6529123
- DOI: 10.1111/cpr.12315
All-trans retinoic acid preconditioning enhances proliferation, angiogenesis and migration of mesenchymal stem cell in vitro and enhances wound repair in vivo
Abstract
Objectives: Stem cell therapy is considered to be a suitable alternative in treatment of a number of diseases. However, there are challenges in their clinical application in cell therapy, such as to reduce survival and loss of transplanted stem cells. It seems that chemical and pharmacological preconditioning enhances their therapeutic efficacy. In this study, we investigated effects of all-trans retinoic acid (ATRA) on survival, angiogenesis and migration of mesenchymal stem cells (MSCs) in vitro and in a wound-healing model.
Materials and methods: MSCs were treated with a variety of concentrations of ATRA, and mRNA expression of cyclo-oxygenase-2 (COX-2), hypoxia-inducible factor-1 (HIF-1), C-X-C chemokine receptor type 4 (CXCR4), C-C chemokine receptor type 2 (CCR2), vascular endothelial growth factor (VEGF), angiopoietin-2 (Ang-2) and Ang-4 were examined by qRT-PCR. Prostaglandin E2 (PGE2) levels were measured using an ELISA kit and MSC angiogenic potential was evaluated using three-dimensional tube formation assay. Finally, benefit of ATRA-treated MSCs in wound healing was determined with a rat excisional wound model.
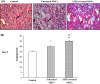
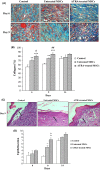
Results: In ATRA-treated MSCs, expressions of COX-2, HIF-1, CXCR4, CCR2, VEGF, Ang-2 and Ang-4 increased compared to control groups. Overexpression of the related genes was reversed by celecoxib, a selective COX-2 inhibitor. Tube formation and in vivo wound healing of ATRA-treated MSCs were also significantly enhanced compared to untreated MSCs.
Conclusion: Pre-conditioning of MSCs with ATRA increased efficacy of cell therapy by activation of survival signalling pathways, trophic factors and release of pro-angiogenic molecules.
Keywords: All-trans retinoic acid; cyclooxygenase-2; mesenchymal stem cells; prostaglandin E2; stem cell therapy.
© 2016 John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interests related to this study.
Figures






References
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

