Effects of covalent modification by 4-hydroxy-2-nonenal on the noncovalent oligomerization of ubiquitin
- PMID: 27862610
- PMCID: PMC5360464
- DOI: 10.1002/jms.3897
Effects of covalent modification by 4-hydroxy-2-nonenal on the noncovalent oligomerization of ubiquitin
Abstract
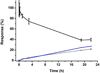
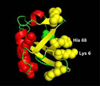
When lipid membranes containing ω-6 polyunsaturated fatty acyl chains are subjected to oxidative stress, one of the reaction products is 4-hydroxy-2-nonenal (HNE)-a chemically reactive short chain alkenal that can covalently modify proteins. The ubiquitin proteasome system is involved in the clearing of proteins modified by oxidation products such as HNE, but the chemical structure, stability and function of ubiquitin may be impaired by HNE modification. To evaluate this possibility, the susceptibility of ubiquitin to modification by HNE has been characterized over a range of concentrations where ubiquitin forms non-covalent oligomers. Results indicate that HNE modifies ubiquitin at only two of the many possible sites, and that HNE modification at these two sites alters the ubiquitin oligomerization equilibrium. These results suggest that any role ubiquitin may have in clearing proteins damaged by oxidative stress may itself be impaired by oxidative lipid degradation products. Copyright © 2016 John Wiley & Sons, Ltd.
Keywords: 4-hydroxy-2-nonenal; Alzheimer's disease; oxidative stress; photocrosslinking; tandem mass spectrometry; ubiquitin.
Copyright © 2016 John Wiley & Sons, Ltd.
Conflict of interest statement
The authors declared no conflicts of interest
Figures



Similar articles
-
Mass spectrometry-based proteomics of oxidative stress: Identification of 4-hydroxy-2-nonenal (HNE) adducts of amino acids using lysozyme and bovine serum albumin as model proteins.Electrophoresis. 2016 Oct;37(20):2615-2623. doi: 10.1002/elps.201600134. Epub 2016 Jun 7. Electrophoresis. 2016. PMID: 27184861 Free PMC article.
-
Low molar excess of 4-oxo-2-nonenal and 4-hydroxy-2-nonenal promote oligomerization of alpha-synuclein through different pathways.Free Radic Biol Med. 2017 Sep;110:421-431. doi: 10.1016/j.freeradbiomed.2017.07.004. Epub 2017 Jul 6. Free Radic Biol Med. 2017. PMID: 28690195
-
Detoxification of cytotoxic alpha,beta-unsaturated aldehydes by carnosine: characterization of conjugated adducts by electrospray ionization tandem mass spectrometry and detection by liquid chromatography/mass spectrometry in rat skeletal muscle.J Mass Spectrom. 2002 Dec;37(12):1219-28. doi: 10.1002/jms.381. J Mass Spectrom. 2002. PMID: 12489081
-
Mass spectrometry for detection of 4-hydroxy-trans-2-nonenal (HNE) adducts with peptides and proteins.Mass Spectrom Rev. 2004 Jul-Aug;23(4):281-305. doi: 10.1002/mas.10076. Mass Spectrom Rev. 2004. PMID: 15133838 Review.
-
Chemistry and Reactivity of 4-hydroxy-2-nonenal (HNE) in Model Biological Systems.Mini Rev Med Chem. 2021;21(12):1394-1405. doi: 10.2174/1389557521666210105110538. Mini Rev Med Chem. 2021. PMID: 33402082 Review.
Cited by
-
Oxidative Stress and 4-hydroxy-2-nonenal (4-HNE): Implications in the Pathogenesis and Treatment of Aging-related Diseases.J Immunol Res. 2022 Mar 23;2022:2233906. doi: 10.1155/2022/2233906. eCollection 2022. J Immunol Res. 2022. PMID: 35411309 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

