Pro-apoptotic Noxa is involved in ablative focal irradiation-induced lung injury
- PMID: 27862899
- PMCID: PMC5345661
- DOI: 10.1111/jcmm.13014
Pro-apoptotic Noxa is involved in ablative focal irradiation-induced lung injury
Abstract
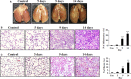
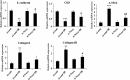
Although lung injury including fibrosis is a well-documented side effect of lung irradiation, the mechanisms underlying its pathology are poorly understood. X-rays are known to cause apoptosis in the alveolar epithelial cells of irradiated lungs, which results in fibrosis due to the proliferation and differentiation of fibroblasts and the deposition of collagen. Apoptosis and BH3-only pro-apoptotic proteins have been implicated in the pathogenesis of pulmonary fibrosis. Recently, we have established a clinically analogous experimental model that reflects focal high-dose irradiation of the ipsilateral lung. The goal of this study was to elucidate the mechanism underlying radiation-induced lung injury based on this model. A radiation dose of 90 Gy was focally delivered to the left lung of C57BL/6 mice for 14 days. About 9 days after irradiation, the mice began to show increased levels of the pro-apoptotic protein Noxa in the irradiated lung alongside increased apoptosis and fibrosis. Suppression of Noxa expression by small interfering RNA protected cells from radiation-induced cell death and decreased expression of fibrogenic markers. Furthermore, we showed that reactive oxygen species participate in Noxa-mediated, radiation-induced cell death. Taken together, our results show that Noxa is involved in X-ray-induced lung injury.
Keywords: Noxa; apoptosis; lung injury; radiation.
© 2016 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures





Similar articles
-
A p38(MAPK)/HIF-1 pathway initiated by UVB irradiation is required to induce Noxa and apoptosis of human keratinocytes.J Invest Dermatol. 2010 Sep;130(9):2269-76. doi: 10.1038/jid.2010.93. Epub 2010 Apr 15. J Invest Dermatol. 2010. PMID: 20393480
-
Systemic polyethylene glycol-modified (PEGylated) superoxide dismutase and catalase mixture attenuates radiation pulmonary fibrosis in the C57/bl6 mouse.Radiother Oncol. 2006 Nov;81(2):196-205. doi: 10.1016/j.radonc.2006.09.013. Epub 2006 Oct 27. Radiother Oncol. 2006. PMID: 17069914 Free PMC article.
-
Blueberry anthocyanins ameliorate radiation-induced lung injury through the protein kinase RNA-activated pathway.Chem Biol Interact. 2015 Dec 5;242:363-71. doi: 10.1016/j.cbi.2015.11.001. Epub 2015 Nov 6. Chem Biol Interact. 2015. PMID: 26551926
-
Apoptosis in lung injury and fibrosis.Eur Respir J. 2008 Dec;32(6):1631-8. doi: 10.1183/09031936.00176807. Eur Respir J. 2008. PMID: 19043009 Review.
-
The Cellular and Molecular Mechanism of Radiation-Induced Lung Injury.Med Sci Monit. 2017 Jul 15;23:3446-3450. doi: 10.12659/msm.902353. Med Sci Monit. 2017. PMID: 28710886 Free PMC article. Review.
Cited by
-
Intercellular transfer of miR-200c-3p impairs the angiogenic capacity of cardiac endothelial cells.Mol Ther. 2022 Jun 1;30(6):2257-2273. doi: 10.1016/j.ymthe.2022.03.002. Epub 2022 Mar 9. Mol Ther. 2022. PMID: 35278675 Free PMC article.
-
Effects of retinoic acid-inducible gene-I-like receptors activations and ionizing radiation cotreatment on cytotoxicity against human non-small cell lung cancer in vitro.Oncol Lett. 2018 Apr;15(4):4697-4705. doi: 10.3892/ol.2018.7867. Epub 2018 Jan 26. Oncol Lett. 2018. PMID: 29541243 Free PMC article.
-
Vitamin D Receptor Protects against Radiation-Induced Intestinal Injury in Mice via Inhibition of Intestinal Crypt Stem/Progenitor Cell Apoptosis.Nutrients. 2021 Aug 24;13(9):2910. doi: 10.3390/nu13092910. Nutrients. 2021. PMID: 34578802 Free PMC article.
-
Identification of the shared genes and immune signatures between systemic lupus erythematosus and idiopathic pulmonary fibrosis.Hereditas. 2023 Mar 4;160(1):9. doi: 10.1186/s41065-023-00270-3. Hereditas. 2023. PMID: 36871016 Free PMC article.
-
PM014 attenuates radiation-induced pulmonary fibrosis via regulating NF-kB and TGF-b1/NOX4 pathways.Sci Rep. 2020 Sep 30;10(1):16112. doi: 10.1038/s41598-020-72629-9. Sci Rep. 2020. PMID: 32999298 Free PMC article.
References
-
- Koto M, Takai Y, Ogawa Y, et al A phase II study on stereotactic body radiotherapy for stage I non‐small cell lung cancer. Radiother Oncol. 2007; 85: 429–34. - PubMed
-
- Timmerman R, Abdulrahman R, Kavanagh BD, et al Lung cancer: a model for implementing stereotactic body radiation therapy into practice. Front Radiat Ther Oncol. 2007; 40: 368–85. - PubMed
-
- Pfister DG, Johnson DH, Azzoli CG, et al American Society of Clinical Oncology treatment of unresectable non‐small‐cell lung cancer guideline: update 2003. J Clin Oncol. 2004; 22: 330–53. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

