Scaffold-free tissue engineering of functional corneal stromal tissue
- PMID: 27863068
- PMCID: PMC5432418
- DOI: 10.1002/term.2363
Scaffold-free tissue engineering of functional corneal stromal tissue
Abstract
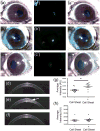
Blinding corneal scarring is predominately treated with allogeneic graft tissue; however, there is a worldwide shortage of donor tissue leaving millions in need of therapy. Human corneal stromal stem cells (CSSC) have been shown produce corneal tissue when cultured on nanofibre scaffolding, but this tissue cannot be readily separated from the scaffold. In this study, scaffold-free tissue engineering methods were used to generate biomimetic corneal stromal tissue constructs that can be transplanted in vivo without introducing the additional variables associated with exogenous scaffolding. CSSC were cultured on substrates with aligned microgrooves, which directed parallel cell alignment and matrix organization, similar to the organization of native corneal stromal lamella. CSSC produced sufficient matrix to allow manual separation of a tissue sheet from the grooved substrate. These constructs were cellular and collagenous tissue sheets, approximately 4 μm thick and contained extracellular matrix molecules typical of corneal tissue including collagen types I and V and keratocan. Similar to the native corneal stroma, the engineered corneal tissues contained long parallel collagen fibrils with uniform diameter. After being transplanted into mouse corneal stromal pockets, the engineered corneal stromal tissues became transparent, and the human CSSCs continued to express human corneal stromal matrix molecules. Both in vitro and in vivo, these scaffold-free engineered constructs emulated stromal lamellae of native corneal stromal tissues. Scaffold-free engineered corneal stromal constructs represent a novel, potentially autologous, cell-generated, biomaterial with the potential for treating corneal blindness. Copyright © 2016 John Wiley & Sons, Ltd.
Keywords: cell sheet; cornea; human cells; ocular; scaffold-free; stem cells; transplantation.
Copyright © 2016 John Wiley & Sons, Ltd.
Figures




References
-
- Levin LA, Adler FH. Adler’s physiology of the eye : clinical application. 11. Edingburg: Saunders/Elsevier; 2011.
-
- Smolin G, Foster CS, Azar DT, Dohlman CH. Smolin and Thoft’s the cornea : scientific foundations and clinical practice. 4. Philadelphia: Lippincott Williams & Wilkins; 2005.
-
- Fini ME. Keratocyte and fibroblast phenotypes in the repairing cornea. Progress in retinal and eye research. 1999;18:529–51. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

