Using Model-Based "Learn and Confirm" to Reveal the Pharmacokinetics-Pharmacodynamics Relationship of Pembrolizumab in the KEYNOTE-001 Trial
- PMID: 27863143
- PMCID: PMC5270295
- DOI: 10.1002/psp4.12132
Using Model-Based "Learn and Confirm" to Reveal the Pharmacokinetics-Pharmacodynamics Relationship of Pembrolizumab in the KEYNOTE-001 Trial
Abstract
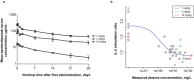
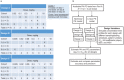
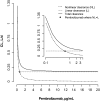
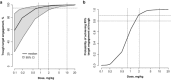
Evaluation of pharmacokinetic/pharmacodynamic (PK/PD) properties played an important role in the early clinical development of pembrolizumab. Because analysis of data from a traditional 3 + 3 dose-escalation design revealed several critical uncertainties, a model-based approach was implemented to better understand these properties. Based on anticipated scenarios for potency and PK nonlinearity, a follow-up study was designed and thoroughly evaluated. Execution of 14,000 virtual trials led to the selection and implementation of a robust design that extended the low-dose range by 200-fold. Modeling of the resulting data demonstrated that pembrolizumab PKs are nonlinear at <0.3 mg/kg every 3 weeks, but linear in the clinical dose range. Saturation of ex vivo target engagement in blood began at ≥1 mg/kg every 3 weeks, and a steady-state dose of 2 mg/kg every 3 weeks was needed to reach 95% target engagement, supporting examination of 2 mg/kg every 3 weeks in ongoing trials in melanoma and other advanced cancers.
© 2016 The Authors CPT: Pharmacometrics & Systems Pharmacology published by Wiley Periodicals, Inc. on behalf of American Society for Clinical Pharmacology and Therapeutics.
Figures

References
-
- Nishimura, H. , Nose, M. , Hiai, H. , Minato, N. & Honjo, T. Development of lupus‐like autoimmune diseases by disruption of the PD‐1 gene encoding an ITIM motif‐carrying immunoreceptor. Immunity 11, 141–151 (1999). - PubMed
-
- Drake, C.G. , Jaffee, E. & Pardoll, D.M. Mechanisms of immune evasion by tumors. Adv. Immunol. 90, 51–81 (2006). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

