Building confidence in quantitative systems pharmacology models: An engineer's guide to exploring the rationale in model design and development
- PMID: 27863172
- PMCID: PMC5351409
- DOI: 10.1002/psp4.12157
Building confidence in quantitative systems pharmacology models: An engineer's guide to exploring the rationale in model design and development
Abstract
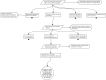
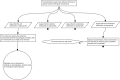
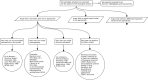
This tutorial promotes good practice for exploring the rationale of systems pharmacology models. A safety systems engineering inspired notation approach provides much needed rigor and transparency in development and application of models for therapeutic discovery and design of intervention strategies. Structured arguments over a model's development, underpinning biological knowledge, and analyses of model behaviors are constructed to determine the confidence that a model is fit for the purpose for which it will be applied.
© 2016 The Authors CPT: Pharmacometrics & Systems Pharmacology published by Wiley Periodicals, Inc. on behalf of American Society for Clinical Pharmacology and Therapeutics.
Figures





References
-
- Kelly, T.P. Arguing safety a systematic approach to managing safety cases. PhD thesis, University of York, YCST 99/05 (1999).
-
- Chinneck, P. , Pumfrey, D. & McDermid, J. The HEAT/ACT preliminary safety case: a case study in the use of goal structuring notation. <http://crpit.com/abstracts/CRPITV47Chinneck.html> (Darlinghurst, Australia, Australia, 2004).
-
- Denney, E. , Pai, G. & Habli, I. Towards measurement of confidence in safety cases. <http://ieeexplore.ieee.org/document/6092593/?arnumber=6092593> (2011).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

