Model-Based Characterization of the Pharmacokinetics of Pembrolizumab: A Humanized Anti-PD-1 Monoclonal Antibody in Advanced Solid Tumors
- PMID: 27863186
- PMCID: PMC5270291
- DOI: 10.1002/psp4.12139
Model-Based Characterization of the Pharmacokinetics of Pembrolizumab: A Humanized Anti-PD-1 Monoclonal Antibody in Advanced Solid Tumors
Abstract
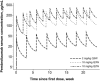
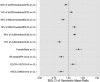
Pembrolizumab, a potent antibody against programmed death 1 (PD-1) receptor, has shown robust antitumor activity and manageable safety in patients with advanced solid tumors. Its pharmacokinetic (PK) properties were analyzed with population PK modeling using pooled data from the KEYNOTE-001, -002, and -006 studies of patients with advanced melanoma, non-small cell lung cancer (NSCLC), and other solid tumor types. Pembrolizumab clearance was low and the volume of distribution small, as is typical for therapeutic antibodies. Identified effects of sex, baseline Eastern Cooperative Oncology Group performance status, measures of renal and hepatic function, tumor type and burden, and prior ipilimumab treatment on pembrolizumab exposure were modest and lacked clinical significance. Furthermore, simulations demonstrated the model has robust power to detect clinically relevant covariate effects on clearance. These results support the use of the approved pembrolizumab dose of 2 mg/kg every 3 weeks without dose adjustment in a variety of patient subpopulations.
© 2016 The Authors CPT: Pharmacometrics & Systems Pharmacology published by Wiley Periodicals, Inc. on behalf of American Society for Clinical Pharmacology and Therapeutics.
Figures

References
-
- Robert, C. et al Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 364, 2517–2526 (2011). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

