The C. elegans Taste Receptor Homolog LITE-1 Is a Photoreceptor
- PMID: 27863243
- PMCID: PMC5388352
- DOI: 10.1016/j.cell.2016.10.053
The C. elegans Taste Receptor Homolog LITE-1 Is a Photoreceptor
Erratum in
-
The C. elegans Taste Receptor Homolog LITE-1 Is a Photoreceptor.Cell. 2017 Jan 12;168(1-2):325. doi: 10.1016/j.cell.2016.12.040. Epub 2017 Jan 12. Cell. 2017. PMID: 28086096 No abstract available.
Abstract
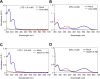
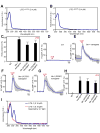
Many animal tissues/cells are photosensitive, yet only two types of photoreceptors (i.e., opsins and cryptochromes) have been discovered in metazoans. The question arises as to whether unknown types of photoreceptors exist in the animal kingdom. LITE-1, a seven-transmembrane gustatory receptor (GR) homolog, mediates UV-light-induced avoidance behavior in C. elegans. However, it is not known whether LITE-1 functions as a chemoreceptor or photoreceptor. Here, we show that LITE-1 directly absorbs both UVA and UVB light with an extinction coefficient 10-100 times that of opsins and cryptochromes, indicating that LITE-1 is highly efficient in capturing photons. Unlike typical photoreceptors employing a prosthetic chromophore to capture photons, LITE-1 strictly depends on its protein conformation for photon absorption. We have further identified two tryptophan residues critical for LITE-1 function. Interestingly, unlike GPCRs, LITE-1 adopts a reversed membrane topology. Thus, LITE-1, a taste receptor homolog, represents a distinct type of photoreceptor in the animal kingdom.
Keywords: chemosensation; chemosensory; neuron; photopigment; photosensation; photosensory.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures





References
-
- Christensen M, Estevez A, Yin X, Fox R, Morrison R, McDonnell M, Gleason C, Miller DM, 3rd, Strange K. A primary culture system for functional analysis of C. elegans neurons and muscle cells. Neuron. 2002;33:503–514. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

