The Allelic Landscape of Human Blood Cell Trait Variation and Links to Common Complex Disease
- PMID: 27863252
- PMCID: PMC5300907
- DOI: 10.1016/j.cell.2016.10.042
The Allelic Landscape of Human Blood Cell Trait Variation and Links to Common Complex Disease
Abstract
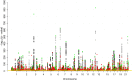
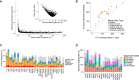
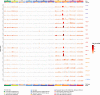
Many common variants have been associated with hematological traits, but identification of causal genes and pathways has proven challenging. We performed a genome-wide association analysis in the UK Biobank and INTERVAL studies, testing 29.5 million genetic variants for association with 36 red cell, white cell, and platelet properties in 173,480 European-ancestry participants. This effort yielded hundreds of low frequency (<5%) and rare (<1%) variants with a strong impact on blood cell phenotypes. Our data highlight general properties of the allelic architecture of complex traits, including the proportion of the heritable component of each blood trait explained by the polygenic signal across different genome regulatory domains. Finally, through Mendelian randomization, we provide evidence of shared genetic pathways linking blood cell indices with complex pathologies, including autoimmune diseases, schizophrenia, and coronary heart disease and evidence suggesting previously reported population associations between blood cell indices and cardiovascular disease may be non-causal.
Keywords: Mendelian randomization; autoimmune diseases; blood; cardiovascular diseases; complex disease; epigenetics; genetics; hematology; hematopoiesis.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures






Comment in
-
Concerted Genetic Function in Blood Traits.Cell. 2016 Nov 17;167(5):1167-1169. doi: 10.1016/j.cell.2016.10.055. Cell. 2016. PMID: 27863238
References
-
- Adams P.C., Reboussin D.M., Barton J.C., McLaren C.E., Eckfeldt J.H., McLaren G.D., Dawkins F.W., Acton R.T., Harris E.L., Gordeuk V.R., Hemochromatosis and Iron Overload Screening (HEIRS) Study Research Investigators Hemochromatosis and iron-overload screening in a racially diverse population. N. Engl. J. Med. 2005;352:1769–1778. - PubMed
-
- Asleh R., Guetta J., Kalet-Litman S., Miller-Lotan R., Levy A.P. Haptoglobin genotype- and diabetes-dependent differences in iron-mediated oxidative stress in vitro and in vivo. Circ. Res. 2005;96:435–441. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

