Kallikrein-related peptidase 6 induces chemotherapeutic resistance by attenuating auranofin-induced cell death through activation of autophagy in gastric cancer
- PMID: 27863404
- PMCID: PMC5356740
- DOI: 10.18632/oncotarget.13352
Kallikrein-related peptidase 6 induces chemotherapeutic resistance by attenuating auranofin-induced cell death through activation of autophagy in gastric cancer
Abstract
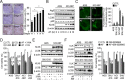
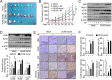
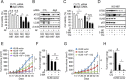
Kallikrein-related peptidase 6 (KLK6) is a biomarker of gastric cancer associated with poor prognosis. Mechanisms by which KLK6 could be exploited for chemotherapeutic use are unclear. We evaluated auranofin (AF), a compound with cytotoxic effects, in KLK6-deficient cells, and we investigated whether KLK6 expression induces autophagy and acquisition of drug resistance in gastric cancer. Using cultured human cells and a mouse xenograft model, we investigated how KLK6 affects antitumor-reagent-induced cell death and autophagy. Expression levels of KLK6, p53, and autophagy marker LC3B were determined in gastric cancer tissues. We analyzed the effects of knockdown/overexpression of KLK6, LC3B, and p53 on AF-induced cell death in cancer cells. Increased KLK6 expression in human gastric cancer tissues and cells inhibited AF-induced cell motility due to increased autophagy and p53 levels. p53 dependent induction of KLK6 expression increased autophagy and drug resistance, whereas KLK6 silencing decreased the autophagy level and increased drug sensitivity. During AF-induced cell death, KLK6 and LC3B colocalized to autophagosomes, associated with p53, and were then trafficked to the cytosol. In the xenograft model of gastric cancer, KLK6 expression decreased AF-induced cell death and KLK6-induced autophagy increased AF resistance. Taken together, the data suggest that the induction of autophagic processes through KLK6 expression may increase acquisition of resistance to AF. Our findings may contribute to a new paradigm for tumor therapeutics.
Keywords: auranofin; autophagy; cell death; chemoresistance; kallikrein-related peptidase 6.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
ING5 suppresses proliferation, apoptosis, migration and invasion, and induces autophagy and differentiation of gastric cancer cells: a good marker for carcinogenesis and subsequent progression.Oncotarget. 2015 Aug 14;6(23):19552-79. doi: 10.18632/oncotarget.3735. Oncotarget. 2015. PMID: 25980581 Free PMC article.
-
Upregulation and secretion of kallikrein-related peptidase 6 (KLK6) in gastric cancer.Tumour Biol. 2012 Jun;33(3):731-8. doi: 10.1007/s13277-011-0267-1. Epub 2012 Feb 29. Tumour Biol. 2012. PMID: 22373580
-
Cystatin SN inhibits auranofin-induced cell death by autophagic induction and ROS regulation via glutathione reductase activity in colorectal cancer.Cell Death Dis. 2017 Mar 16;8(3):e2682. doi: 10.1038/cddis.2017.100. Cell Death Dis. 2017. PMID: 28300829 Free PMC article.
-
Molecular Mechanism of Resistance to Chemotherapy in Gastric Cancers, the Role of Autophagy.Curr Drug Targets. 2020;21(7):713-721. doi: 10.2174/1389450120666191127113854. Curr Drug Targets. 2020. PMID: 31775598 Review.
-
Targeting autophagy to overcome drug resistance: further developments.J Hematol Oncol. 2020 Nov 25;13(1):159. doi: 10.1186/s13045-020-01000-2. J Hematol Oncol. 2020. PMID: 33239065 Free PMC article. Review.
Cited by
-
A risk model based on autophagy-related lncRNAs for predicting prognosis and efficacy of immunotherapy and chemotherapy in gastric cancer patients.Aging (Albany NY). 2021 Dec 12;13(23):25453-25465. doi: 10.18632/aging.203765. Epub 2021 Dec 12. Aging (Albany NY). 2021. PMID: 34897033 Free PMC article.
-
Gold-based therapy: From past to present.Proc Natl Acad Sci U S A. 2020 Sep 15;117(37):22639-22648. doi: 10.1073/pnas.2007285117. Epub 2020 Sep 8. Proc Natl Acad Sci U S A. 2020. PMID: 32900936 Free PMC article. Review.
-
Thioredoxin Confers Intrinsic Resistance to Cytostatic Drugs in Human Glioma Cells.Int J Mol Sci. 2018 Sep 21;19(10):2874. doi: 10.3390/ijms19102874. Int J Mol Sci. 2018. PMID: 30248944 Free PMC article.
-
Silencing of kallikrein-related peptidase 6 attenuates the proliferation, migration, and invasion of gastric cancer cells through inhibition of epithelial-mesenchymal transition.Exp Ther Med. 2021 Jul;22(1):770. doi: 10.3892/etm.2021.10202. Epub 2021 May 17. Exp Ther Med. 2021. PMID: 34055069 Free PMC article.
-
Wheat germ agglutinin-induced paraptosis-like cell death and protective autophagy is mediated by autophagy-linked FYVE inhibition.Oncotarget. 2017 Aug 24;8(53):91209-91222. doi: 10.18632/oncotarget.20436. eCollection 2017 Oct 31. Oncotarget. 2017. PMID: 29207637 Free PMC article.
References
-
- Turner R. Gastric cancer gets the run-around. Nat Med. 2002;8:449. - PubMed
-
- Fan K, Fan D, Cheng LF, Li C. Expression of multidrug resistance-related markers in gastric cancer. Anticancer Res. 2000;20:4809–14. - PubMed
-
- Zhou J, Yong WP, Yap CS, Vijayaraghavan A, Sinha RA, Singh BK, Xiu S, Manesh S, Ngo A, Lim A, Ang C, Xie C, et al. An integrative approach identified genes associated with drug response in gastric cancer. Carcinogenesis. 2015;36:441–51. - PubMed
-
- Gray-Schopfer VC, Karasarides M, Hayward R, Marais R. Tumor necrosis factor-a blocks apoptosis in melanoma cells when BRAF signaling is inhibited. Cancer Res. 2007;67:122–9. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

