Epigenetically upregulated WIPF1 plays a major role in BRAF V600E-promoted papillary thyroid cancer aggressiveness
- PMID: 27863429
- PMCID: PMC5352205
- DOI: 10.18632/oncotarget.13400
Epigenetically upregulated WIPF1 plays a major role in BRAF V600E-promoted papillary thyroid cancer aggressiveness
Abstract
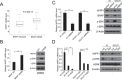
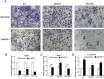
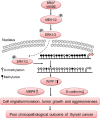
How the BRAF V600E mutation promotes the pathogenesis and aggressiveness of papillary thyroid cancer (PTC) is not completely understood. Here we explored a novel mechanism involving WASP interacting protein family member 1 (WIPF1). In PTC tumors, compared with the wild-type BRAF, BRAF V600E was associated with over-expression and hypomethylation of the WIPF1 gene. In thyroid cancer cell lines with wild-type BRAF, WIPF1 expression was robustly upregulated upon introduced expression of BRAF V600E (P=0.03) whereas the opposite was seen upon BRAF knockdown or treatment with BRAF V600E or MEK inhibitors in cells harboring BRAF V600E. Methylation of a functionally critical region of the WIPF1 promoter was decreased by expressing BRAF V600E in cells harboring the wild-type BRAF and increased by BRAF knockdown or treatment with BRAF V600E or MEK inhibitors in cells harboring BRAF V600E mutation. Under-expression and hypermethylation of WIPF1 induced by stable BRAF knockdown was reversed by DNA demethylating agent 5'-azadeoxycytidine. Knockdown of WIPF1 robustly inhibited anchorage-independent colony formation, migration, and invasion of thyroid cancer cells and suppressed xenograft thyroid cancer tumor growth and vascular invasion, mimicking the effects of BRAF knockdown. In human PTC tumors, WIPF1 expression was associated with extrathyroidal invasion (P=0.01) and lymph node metastasis (P=2.64E-05). In summary, BRAF V600E-activated MAP kinase pathway causes hypomethylation and overexpression of WIPF1; WIPF1 then functions like an oncoprotein to robustly promote aggressive cellular and tumor behaviors of PTC. This represents a novel mechanism in BRAF V600E-promoted PTC aggressiveness and identifies WIPF1 as a novel therapeutic target for thyroid cancer.
Keywords: BRAF V600E; WIPF1; oncogenesis; thyroid cancer; tumor aggressiveness.
Conflict of interest statement
M.X. received royalty payments as co-holder of a licensed USA patent related to BRAF V600E mutation in thyroid cancer. No potential conflicts of interest were disclosed by other authors.
Figures





References
-
- Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, et al. SEER Cancer Statistics Review, 1975-2013. National Cancer Institute; Bethesda, MD: Apr, 2016. http://seercancergov/csr/1975_2013/ based on November 2015 SEER data submission, posted to the SEER web site. Accessed on July 5, 2016.
-
- Tuttle RM, Ball DW, Byrd D, Dilawari RA, Doherty GM, Duh QY, Ehya H, Farrar WB, Haddad RI, Kandeel F, Kloos RT, Kopp P, Lamonica DM, et al. Thyroid carcinoma. J Natl Compr Canc Netw. 2010;8:1228–74. - PubMed
-
- Lam AK, Lo CY, Lam KS. Papillary carcinoma of thyroid: A 30-yr clinicopathological review of the histological variants. Endocr Pathol. 2005;16:323–30. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

