Interleukin 37 limits monosodium urate crystal-induced innate immune responses in human and murine models of gout
- PMID: 27863506
- PMCID: PMC5116141
- DOI: 10.1186/s13075-016-1167-y
Interleukin 37 limits monosodium urate crystal-induced innate immune responses in human and murine models of gout
Abstract
Background: Interleukin (IL)-37 has emerged as a fundamental inhibitor of innate immunity. Acute gout is a self-limiting inflammatory response to monosodium urate (MSU) crystals. In the current study, we assessed the preventive and therapeutic effect of recombinant human IL-37 (rhIL-37) in human and murine gout models.
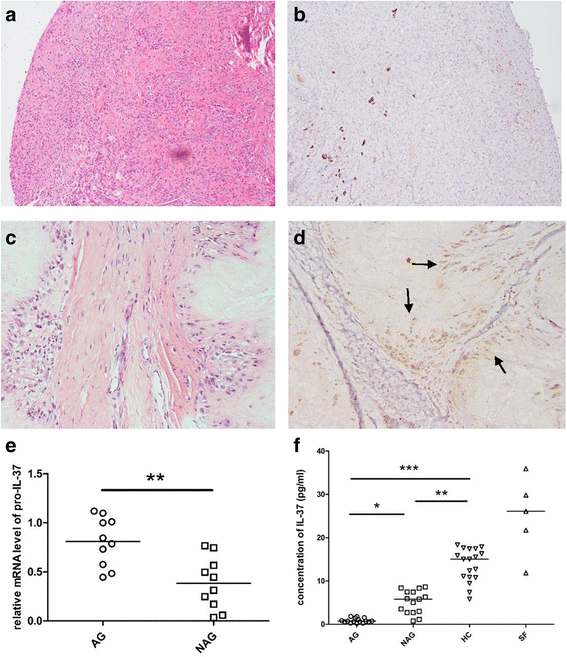
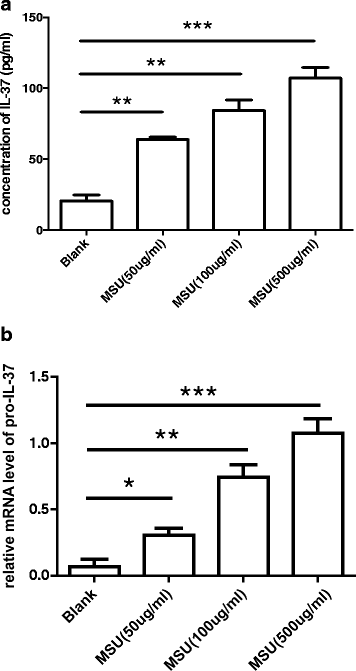
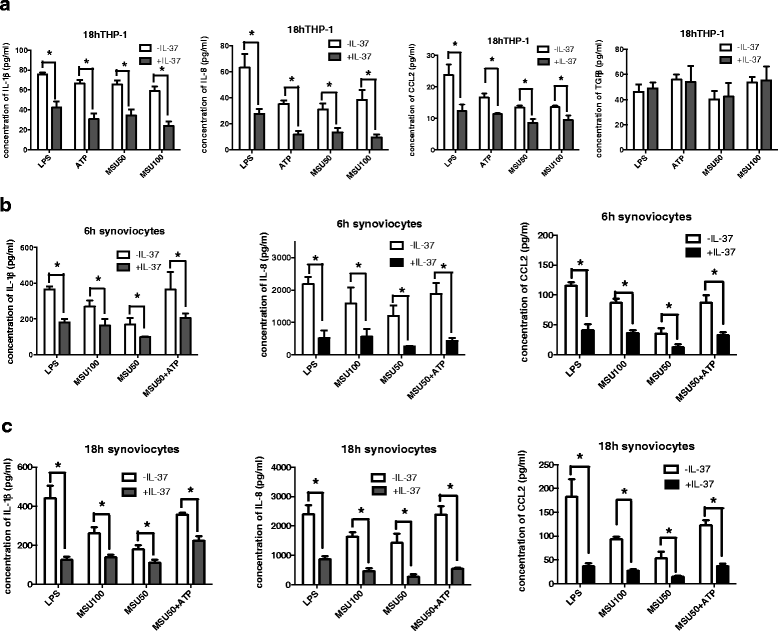
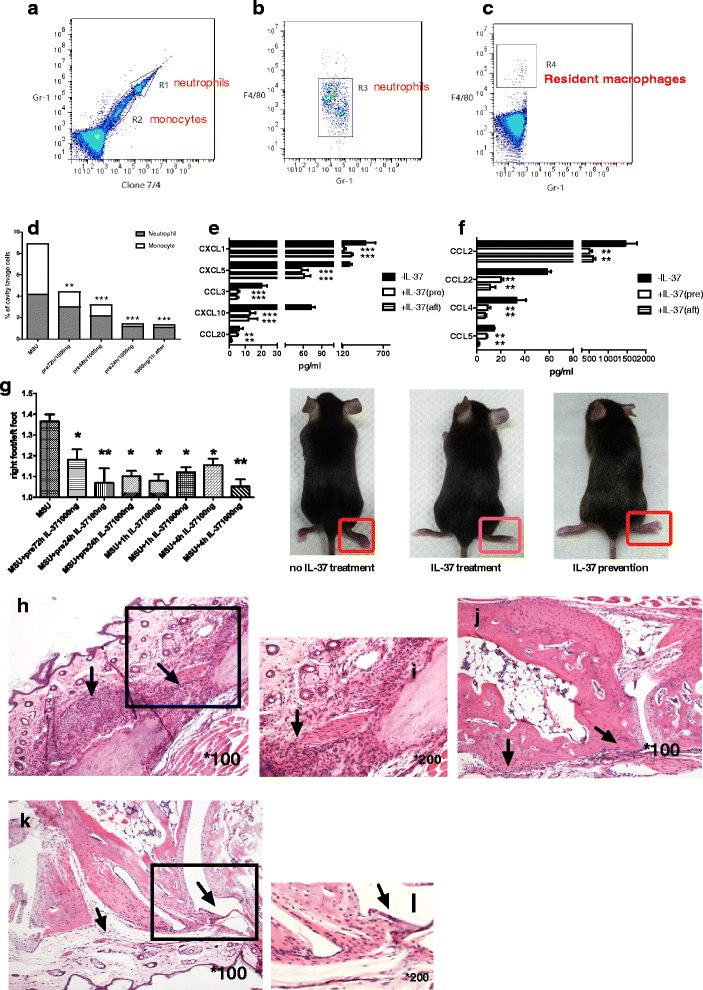
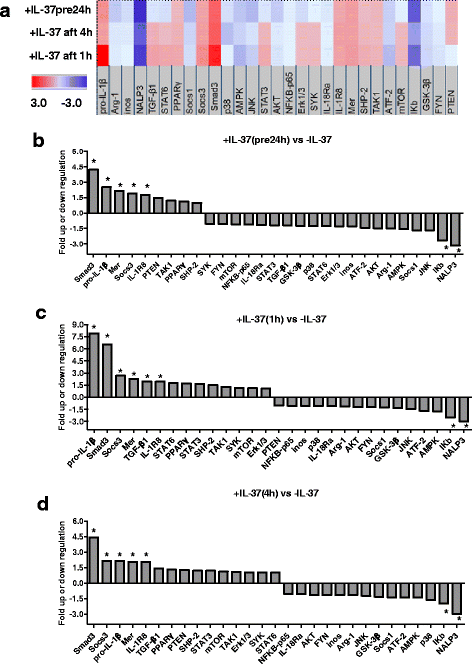
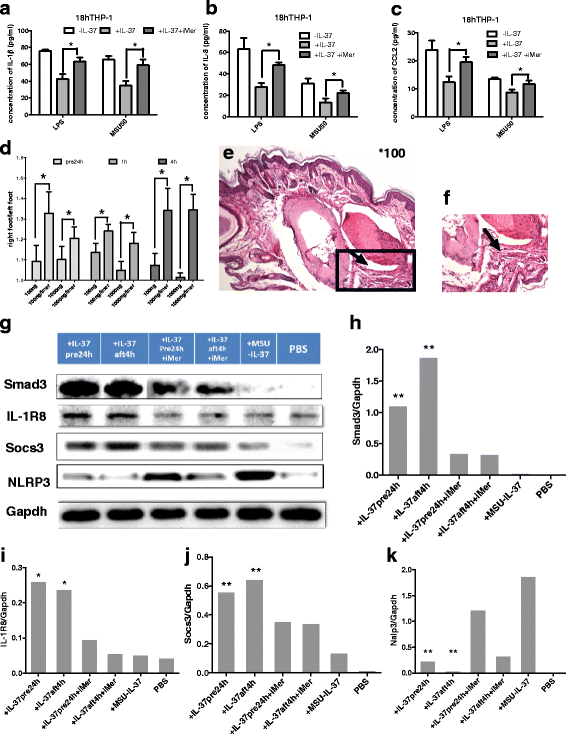
Methods: We investigated the expression of IL-37 in patients with active and inactive gouty arthritis and assessed the effect of rhIL-37 in human and murine gout models: a human monocyte cell line (THP-1) and human synovial cells (containing macrophage-like and fibroblast-like synoviocytes) exposed to MSU crystals, a peritoneal murine model of gout and a murine gouty arthritis model. After inhibition of Mer receptor tyrosine kinase (Mertk), levels of IL-1β, IL-8 and chemokine (C-C motif) ligand 2 (CCL-2) were detected by ELISA and expression of mammalian homologs of the drosophila Mad gene 3 (Smad), suppressor of cytokine signaling 3 (SOCS3), NACHT-LRR-PYD-containing protein 3 (NLRP3), and IL-8R of THP-1 were assessed by qPCR and western blot to explore the molecular mechanisms.
Results: Our studies strongly indicated that rhIL-37 played a potent immunosuppressive role in the pathogenesis of experimental gout models both in vitro and in vivo, by downregulating proinflammatory cytokines and chemokines, markedly reducing neutrophil and monocyte recruitment, and mitigating pathological joint inflammation. In our studies, rhIL-37 suppressed MSU-induced innate immune responses by enhancing expression of Smad3 and IL-1R8 to trigger multiple intracellular switches to block inflammation, including inhibition of NLRP3 and activation of SOCS3. Mertk signaling participated in rhIL-37 inhibitory pathways in gout models. By inhibition of Mertk, the anti-inflammatory effect of rhIL-37 was partly abrogated, and IL-1R8, Smad3 and SOCS3 expression were suppressed, whereas NLRP3 expression was reactivated.
Conclusions: Our studies reveal that IL-37 limits runaway inflammation initiated by MSU crystal-induced immune responses, partly in a Mertk-dependent fashion. Thus, rhIL-37 has both preventive and therapeutic effects in gouty arthritis.
Keywords: Gout; IL-1R8; IL-37; MSU; Mertk; NLRP3; Smad3; SOCS3.
Figures
Similar articles
-
NLRP3 inflammasome-mediated neutrophil recruitment and hypernociception depend on leukotriene B(4) in a murine model of gout.Arthritis Rheum. 2012 Feb;64(2):474-84. doi: 10.1002/art.33355. Arthritis Rheum. 2012. PMID: 21952942
-
Innate immunity conferred by Toll-like receptors 2 and 4 and myeloid differentiation factor 88 expression is pivotal to monosodium urate monohydrate crystal-induced inflammation.Arthritis Rheum. 2005 Sep;52(9):2936-46. doi: 10.1002/art.21238. Arthritis Rheum. 2005. PMID: 16142712
-
Synovial fluid proteins are required for the induction of interleukin-1β production by monosodium urate crystals.Scand J Rheumatol. 2016 Oct;45(5):384-93. doi: 10.3109/03009742.2015.1124452. Epub 2016 May 20. Scand J Rheumatol. 2016. PMID: 27206713
-
Crystal-induced neutrophil activation.Immunol Cell Biol. 2010 Jan;88(1):32-40. doi: 10.1038/icb.2009.98. Epub 2009 Dec 1. Immunol Cell Biol. 2010. PMID: 19949421 Review.
-
The Mechanism of the NLRP3 Inflammasome Activation and Pathogenic Implication in the Pathogenesis of Gout.J Rheum Dis. 2022 Jul 1;29(3):140-153. doi: 10.4078/jrd.2022.29.3.140. J Rheum Dis. 2022. PMID: 37475970 Free PMC article. Review.
Cited by
-
Identification of SOCS3 and PTGS2 as new biomarkers for the diagnosis of gout by cross-species comprehensive analysis.Heliyon. 2024 Apr 23;10(9):e30020. doi: 10.1016/j.heliyon.2024.e30020. eCollection 2024 May 15. Heliyon. 2024. PMID: 38707281 Free PMC article.
-
Biology of interleukin-37 and its role in autoimmune diseases (Review).Exp Ther Med. 2022 Jun 7;24(2):495. doi: 10.3892/etm.2022.11422. eCollection 2022 Aug. Exp Ther Med. 2022. PMID: 35837057 Free PMC article. Review.
-
Interleukin-37 Attenuates Lipopolysaccharide (LPS)-Induced Neonatal Acute Respiratory Distress Syndrome in Young Mice via Inhibition of Inflammation and Cell Apoptosis.Med Sci Monit. 2020 Mar 10;26:e920365. doi: 10.12659/MSM.920365. Med Sci Monit. 2020. PMID: 32152260 Free PMC article.
-
Mechanism of macrophages in gout: Recent progress and perspective.Heliyon. 2024 Sep 21;10(19):e38288. doi: 10.1016/j.heliyon.2024.e38288. eCollection 2024 Oct 15. Heliyon. 2024. PMID: 39386881 Free PMC article. Review.
-
The genetic backbone of ankylosing spondylitis: how knowledge of genetic susceptibility informs our understanding and management of disease.Rheumatol Int. 2022 Dec;42(12):2085-2095. doi: 10.1007/s00296-022-05174-5. Epub 2022 Aug 8. Rheumatol Int. 2022. PMID: 35939079 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

