Effects of continuous renal replacement therapy on linezolid pharmacokinetic/pharmacodynamics: a systematic review
- PMID: 27863531
- PMCID: PMC5116218
- DOI: 10.1186/s13054-016-1551-7
Effects of continuous renal replacement therapy on linezolid pharmacokinetic/pharmacodynamics: a systematic review
Abstract
Background: Major alterations in linezolid pharmacokinetic/pharmacodynamic (PK/PD) parameters might be expected in critically ill septic patients with acute kidney injury (AKI) who are undergoing continuous renal replacement therapy (CRRT). The present review is aimed at describing extracorporeal removal of linezolid and the main PK-PD parameter changes observed in critically ill septic patients with AKI, who are on CRRT.
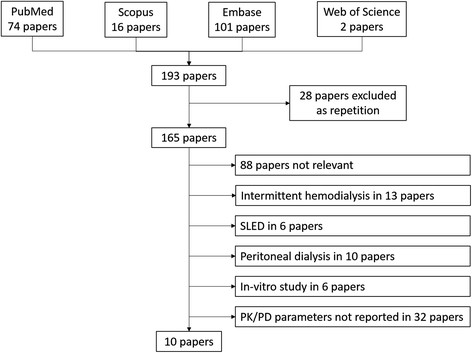
Method: Citations published on PubMed up to January 2016 were systematically reviewed according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement. All authors assessed the methodological quality of the studies and consensus was used to ensure studies met inclusion criteria. In-vivo studies in adult patients with AKI treated with linezolid and on CRRT were considered eligible for the analysis only if operational settings of the CRRT machine, membrane type, linezolid blood concentrations and main PK-PD parameters were all clearly reported.
Results: Among 68 potentially relevant articles, only 9 were considered eligible for the analysis. Across these, 53 treatments were identified among the 49 patients included (46 treated with high-flux and 3 with high cut-off membranes). Continuous veno-venous hemofiltration (CVVH) was the most frequent treatment performed amongst the studies. The extracorporeal clearance values of linezolid across the different modalities were 1.2-2.3 L/h for CVVH, 0.9-2.2 L/h for hemodiafiltration and 2.3 L/h for hemodialysis, and large variability in PK/PD parameters was reported. The optimal area under the curve/minimum inhibitory concentration (AUC/MIC) ratio was reached for pathogens with an MIC of 4 mg/L in one study only.
Conclusions: Wide variability in linezolid PK/PD parameters has been observed across critically ill septic patients with AKI treated with CRRT. Particular attention should be paid to linezolid therapy in order to avoid antibiotic failure in these patients. Strategies to improve the effectiveness of this antimicrobial therapy (such as routine use of target drug monitoring, increased posology or extended infusion) should be carefully evaluated, both in clinical and research settings.
Keywords: Acute kidney injury; Adsorption; Clearance; Critical illness; High cut-off membranes; High-flux membranes; Linezolid; Sepsis.
Figures
References
-
- Kim WY, Huh JW, Lim C-M, Koh Y, Hong S-B. Analysis of progression in risk, injury, failure, loss, and end-stage renal disease classification on outcome in patients with severe sepsis and septic shock. J Crit Care. 2012;27:104.e1–7. - PubMed
-
- Chelazzi C, Pettini E, Villa G, De Gaudio AR. Epidemiology, associated factors and outcomes of ICU-acquired infections caused by Gram-negative bacteria in critically ill patients: an observational, retrospective study. BMC Anesthesiol. 2015;15:125. doi: 10.1186/s12871-015-0106-9. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous