Is it feasible to use granulocyte-colony stimulating factor alone to mobilize progenitor cells in multiple myeloma patients induced with a cyclophosphamide, thalidomide and dexamethasone regimen?
- PMID: 27863757
- PMCID: PMC5119677
- DOI: 10.1016/j.bjhh.2016.06.004
Is it feasible to use granulocyte-colony stimulating factor alone to mobilize progenitor cells in multiple myeloma patients induced with a cyclophosphamide, thalidomide and dexamethasone regimen?
Abstract
Background: Cyclophosphamide plus thalidomide as induction for multiple myeloma patients eligible for autologous stem cell transplantation may be a limiting factor for cell mobilization. The minimum acceptable mobilized peripheral blood stem cell count to prevent deleterious effects during transplantation is 2.0×106 CD34+ cells/kg. Combining other treatments to granulocyte-colony stimulating factor, such as cyclophosphamide, could overcome the mobilization limitation. The objective of this study was to assess the number of CD34+ cells mobilized using granulocyte-colony stimulating factor with and without cyclophosphamide after induction with cyclophosphamide, thalidomide and dexamethasone.
Methods: A retrospective study was performed of a cohort of multiple myeloma patients submitted to autologous stem cell transplantations at two Brazilian centers between May 2009 and July 2013. The oral cyclophosphamide and thalidomide induction doses used were 1500mg/month and 100-200mg/day, respectively. Mobilization doses were 10-15mcg/kg granulocyte-colony stimulating factor with 2-4g/m2 cyclophosphamide, or 15-20mcg/kg granulocyte-colony stimulating factor alone for 5 days. Collection of >2.0×106 CD34+ cells/kg was considered sufficient.
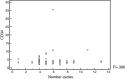
Results: Eighty-eight patients were analyzed; only 18 received cyclophosphamide. The median age was 58 years old (range: 51-62) for the granulocyte-colony stimulating factor group and 56.5 years old (range: 54-60) for granulocyte-colony stimulating factor plus cyclophosphamide group. Fifty-two patients were male. Eighty cases (90.9%) were Durie-Salmon Staging System III-A/B and 38 (44.7%) and 20 cases (23.5%) were International Staging System 2 and 3, respectively. The group that received cyclophosphamide collected a higher median number of progenitor cells [3.8 (range: 3.1-4.4) vs. 3.2 (range: 2.3-3.8)] (p-value=0.008). No correlation was observed between better responses or number of induction cycles and the number of cells collected.
Conclusion: The number of cells mobilized with granulocyte-colony stimulating factor plus cyclophosphamide was higher. However, in both groups, the median number of CD34+ cells was sufficient to perform a single autologous stem cell transplantation; no deleterious effects were reported during harvesting.
Keywords: Bone marrow transplantation; Cyclophosphamide; Granulocyte-colony stimulating factor; Multiple myeloma.
Copyright © 2016 Associação Brasileira de Hematologia, Hemoterapia e Terapia Celular. Published by Elsevier Editora Ltda. All rights reserved.
Figures
References
-
- Auner H.W., Mazzarella L., Cook L., Szydlo R., Saltarelli F., Pavlu J. High rate of stem cell mobilization failure after thalidomide and oral cyclophosphamide induction therapy for multiple myeloma. Bone Marrow Transplant. 2011;46(3):364–367. - PubMed
-
- Desikan K.R., Tricot G., Munshi N.C., Anaissie E., Spoon D., Fassas A. Preceding chemotherapy, tumour load and age influence engraftment in multiple myeloma patients mobilized with granulocyte colony-stimulating factor alone. Br J Haematol. 2001;112(1):242–247. - PubMed
-
- Kumar S., Dispenzieri A., Lacy M.Q., Hayman S.R., Buadi F.K., Gastineau D.A. Impact of lenalidomide therapy on stem cell mobilization and engraftment post-peripheral blood stem cell transplantation in patients with newly diagnosed myeloma. Leukemia. 2007;21(9):2035–2042. - PubMed
-
- Breitkreutz I., Lokhorst H.M., Raab M.S., Holt Bv, Cremer F.W., Herrmann D. Thalidomide in newly diagnosed multiple myeloma: influence of thalidomide treatment on peripheral blood stem cell collection yield. Leukemia. 2007;21(6):1294–1299. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources