The Multibiome: The Intestinal Ecosystem's Influence on Immune Homeostasis, Health, and Disease
- PMID: 27863931
- PMCID: PMC5264270
- DOI: 10.1016/j.ebiom.2016.10.007
The Multibiome: The Intestinal Ecosystem's Influence on Immune Homeostasis, Health, and Disease
Abstract
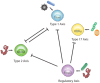
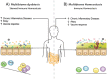
Mammalian evolution has occurred in the presence of mutualistic, commensal, and pathogenic micro- and macro-organisms for millennia. The presence of these organisms during mammalian evolution has allowed for intimate crosstalk between these colonizing species and the host immune system. In this review, we introduce the concept of the 'multibiome' to holistically refer to the biodiverse collection of bacteria, viruses, fungi and multicellular helminthic worms colonizing the mammalian intestine. Furthermore, we discuss new insights into multibiome-host interactions in the context of host-protective immunity and immune-mediated diseases, including inflammatory bowel disease and multiple sclerosis. Finally, we provide reasons to account for the multibiome in experimental design, analysis and in therapeutic applications.
Keywords: Autoimmune disease; Helminth immunotherapy; Inflammatory bowel disease; Microbiome; Mucosal immunology.
Copyright © 2016 The Authors. Published by Elsevier B.V. All rights reserved.
Figures



References
-
- Allen J.E., Maizels R.M. Diversity and dialogue in immunity to helminths. Nat. Rev. Immunol. 2011;11:375–388. - PubMed
-
- Ascherio A., Munger K.L. Epidemiology of multiple sclerosis: from risk factors to prevention-an update. Semin. Neurol. 2016;36:103–114. - PubMed
-
- Basic M., Keubler L.M., Buettner M., Achard M., Breves G., Schroder B., Smoczek A., Jorns A., Wedekind D., Zschemisch N.H., Gunther C., Neumann D., Lienenklaus S., Weiss S., Hornef M.W., Mahler M., Bleich A. Norovirus triggered microbiota-driven mucosal inflammation in interleukin 10-deficient mice. Inflamm. Bowel Dis. 2014;20:431–443. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

