Region-specific role for Pten in maintenance of epithelial phenotype and integrity
- PMID: 27864284
- PMCID: PMC5283927
- DOI: 10.1152/ajplung.00005.2015
Region-specific role for Pten in maintenance of epithelial phenotype and integrity
Abstract
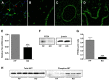
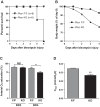
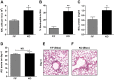
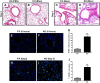
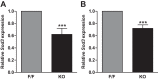
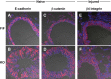
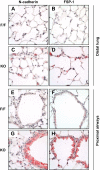
Previous studies have demonstrated resistance to naphthalene-induced injury in proximal airways of mice with lung epithelial-specific deletion of the tumor-suppressor gene Pten, attributed to increased proliferation of airway progenitors. We tested effects of Pten loss following bleomycin injury, a model typically used to study distal lung epithelial injury, in conditional PtenSFTPC-cre knockout mice. Pten-deficient airway epithelium exhibited marked hyperplasia, particularly in small bronchioles and at bronchoalveolar duct junctions, with reduced E-cadherin and β-catenin expression between cells toward the luminal aspect of the hyperplastic epithelium. Bronchiolar epithelial and alveolar epithelial type II (AT2) cells in PtenSFTPC-cre mice showed decreased expression of epithelial markers and increased expression of mesenchymal markers, suggesting at least partial epithelial-mesenchymal transition at baseline. Surprisingly, and in contrast to previous studies, mutant mice were exquisitely sensitive to bleomycin, manifesting rapid weight loss, respiratory distress, increased early mortality (by day 5), and reduced dynamic lung compliance. This was accompanied by sloughing of the hyperplastic airway epithelium with occlusion of small bronchioles by cellular debris, without evidence of increased parenchymal lung injury. Increased airway epithelial cell apoptosis due to loss of antioxidant defenses, reflected by decreased expression of superoxide dismutase 3, in combination with deficient intercellular adhesion, likely predisposed to airway sloughing in knockout mice. These findings demonstrate an important role for Pten in maintenance of airway epithelial phenotype integrity and indicate that responses to Pten deletion in respiratory epithelium following acute lung injury are highly context-dependent and region-specific.
Keywords: adherens junctions; alveolar epithelium; bleomycin; cell adhesion; reactive oxygen species.
Copyright © 2017 the American Physiological Society.
Figures

References
-
- Adamson IY, Bowden DH. The type 2 cell as progenitor of alveolar epithelial regeneration. A cytodynamic study in mice after exposure to oxygen. Lab Invest 30: 35–42, 1974. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

