Mitochondrial and redox abnormalities in autism lymphoblastoid cells: a sibling control study
- PMID: 27864377
- PMCID: PMC5354548
- DOI: 10.1096/fj.201601004R
Mitochondrial and redox abnormalities in autism lymphoblastoid cells: a sibling control study
Abstract
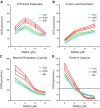
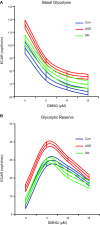
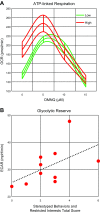
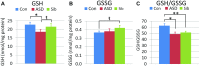
Autism spectrum disorder (ASD) is associated with physiological abnormalities, including abnormal redox and mitochondrial metabolism. Lymphoblastoid cell lines (LCLs) from some children with ASD exhibit increased oxidative stress, decreased glutathione redox capacity, and highly active mitochondria with increased vulnerability to reactive oxygen species (ROS). Because unaffected siblings (Sibs) of individuals with ASD share some redox abnormalities, we sought to determine whether LCLs from Sibs share ASD-associated mitochondrial abnormalities. We evaluated mitochondrial bioenergetics in 10 sets of LCLs from children with ASD, Sibs, and unrelated/unaffected controls (Cons) after acute increases in ROS. Additionally, intracellular glutathione and uncoupling protein 2 (UCP2) gene expressions were quantified. Compared to Sib LCLs, ASD LCLs exhibited significantly higher ATP-linked respiration, higher maximal and reserve respiratory capacity, and greater glycolysis and glycolytic reserve. ASD LCLs exhibited a significantly greater change in these parameters, with acute increases in ROS compared to both Sib and Con LCLs. Compared to Con, both ASD and Sib LCLs exhibited significantly higher proton leak respiration. Consistent with this, intracellular glutathione redox capacity was decreased and UCP2 gene expression was increased in both ASD and Sib compared to Con LCLs. These data indicate that mitochondrial respiratory function, not abnormal redox homeostasis, distinguishes ASD from unaffected LCLs.-Rose, S., Bennuri, S. C., Wynne, R., Melnyk, S., James, S. J., Frye, R. E. Mitochondrial and redox abnormalities in autism lymphoblastoid cells: a sibling control study.
Keywords: UCP2; autistic disorder; bioenergetics; glutathione; oxidative stress.
© FASEB.
Figures

References
-
- Frye R. E. (2012) Biomarker of abnormal energy metabolism in children with autism spectrum disorder. N. Am. J. Med. Sci. (Boston) 5, 141–147
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

