Molecular dynamics of Dkk4 modulates Wnt action and regulates meibomian gland development
- PMID: 27864382
- PMCID: PMC5201035
- DOI: 10.1242/dev.143909
Molecular dynamics of Dkk4 modulates Wnt action and regulates meibomian gland development
Abstract
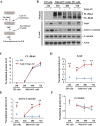
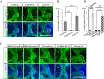
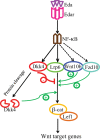
Secreted Dickkopf (Dkk) proteins are major Wnt pathway modulators during organ development. Dkk1 has been widely studied and acts as a general Wnt inhibitor. However, the molecular function of other Dkks remains largely unknown. Here, we show that Dkk4 selectively inhibits a subset of Wnts, but is further inactivated by proteolytic cleavage. Meibomian gland (MG) formation is employed as a model where Dkk4 and its Wnt targets are expressed. Skin-specific expression of Dkk4 arrests MG growth at early germ phase, which is similar to that observed in Eda-ablated Tabby mice. Consistent with transient Dkk4 action, intact Dkk4 inhibits MG extension but the cleaved form progressively increases during MG development with a concomitant upswing in Wnt activity. Furthermore, both Dkk4 and its receptor (and Wnt co-receptor) Lrp6 are direct Eda targets during MG induction. In cell and organotypic cultures, Dkk4 inhibition is eliminated by elevation of Lrp6. Also, Lrp6 upregulation restores MG formation in Tabby mice. Thus, the dynamic state of Dkk4 itself and its interaction with Lrp6 modulates Wnt function during MG development, with a novel limitation of Dkk4 action by proteolytic cleavage.
Keywords: Dkk4; Eda; Lrp6; Meibomian gland; Mouse; Proteolytic cleavage; Wnt.
© 2016. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures

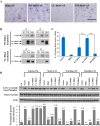
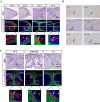
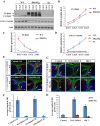
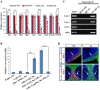
Similar articles
-
Structural and functional analysis of Dickkopf 4 (Dkk4): New insights into Dkk evolution and regulation of Wnt signaling by Dkk and Kremen proteins.J Biol Chem. 2018 Aug 3;293(31):12149-12166. doi: 10.1074/jbc.RA118.002918. Epub 2018 Jun 20. J Biol Chem. 2018. PMID: 29925589 Free PMC article.
-
Involvement of Wnt, Eda and Shh at defined stages of sweat gland development.Development. 2014 Oct;141(19):3752-60. doi: 10.1242/dev.109231. Development. 2014. PMID: 25249463 Free PMC article.
-
Structural basis of Wnt signaling inhibition by Dickkopf binding to LRP5/6.Dev Cell. 2011 Nov 15;21(5):862-73. doi: 10.1016/j.devcel.2011.09.003. Epub 2011 Oct 13. Dev Cell. 2011. PMID: 22000856 Free PMC article.
-
Role of DKK4 in Tumorigenesis and Tumor Progression.Int J Biol Sci. 2018 Apr 26;14(6):616-621. doi: 10.7150/ijbs.24329. eCollection 2018. Int J Biol Sci. 2018. PMID: 29904276 Free PMC article. Review.
-
A literature review on function and regulation mechanism of DKK4.J Cell Mol Med. 2021 Mar;25(6):2786-2794. doi: 10.1111/jcmm.16372. Epub 2021 Feb 14. J Cell Mol Med. 2021. PMID: 33586359 Free PMC article. Review.
Cited by
-
Dickkopf-4 gene expression is associated with differentiation and lymph node metastasis in colorectal cancer.JGH Open. 2019 Apr 10;3(5):409-416. doi: 10.1002/jgh3.12177. eCollection 2019 Oct. JGH Open. 2019. PMID: 31633047 Free PMC article.
-
Dickkopf-4 is frequently overexpressed in epithelial ovarian carcinoma and promotes tumor invasion.BMC Cancer. 2017 Jun 30;17(1):455. doi: 10.1186/s12885-017-3407-1. BMC Cancer. 2017. PMID: 28666421 Free PMC article.
-
[Research advances on signaling pathways affecting sweat gland development and their involvement in the reconstitution of sweat adenoid cells in vitro].Zhonghua Shao Shang Yu Chuang Mian Xiu Fu Za Zhi. 2022 Feb 20;38(2):195-200. doi: 10.3760/cma.j.cn501120-20201020-00442. Zhonghua Shao Shang Yu Chuang Mian Xiu Fu Za Zhi. 2022. PMID: 35220709 Free PMC article. Review. Chinese.
-
Meibomian gland development: Where, when and how?Differentiation. 2023 Jul-Aug;132:41-50. doi: 10.1016/j.diff.2023.04.005. Epub 2023 May 5. Differentiation. 2023. PMID: 37202278 Free PMC article. Review.
-
Eda-activated RelB recruits an SWI/SNF (BAF) chromatin-remodeling complex and initiates gene transcription in skin appendage formation.Proc Natl Acad Sci U S A. 2018 Aug 7;115(32):8173-8178. doi: 10.1073/pnas.1800930115. Epub 2018 Jul 23. Proc Natl Acad Sci U S A. 2018. PMID: 30037996 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

