Thiamet G mediates neuroprotection in experimental stroke by modulating microglia/macrophage polarization and inhibiting NF-κB p65 signaling
- PMID: 27864466
- PMCID: PMC5536801
- DOI: 10.1177/0271678X16679671
Thiamet G mediates neuroprotection in experimental stroke by modulating microglia/macrophage polarization and inhibiting NF-κB p65 signaling
Erratum in
-
Corrigendum.J Cereb Blood Flow Metab. 2022 May;42(5):905-907. doi: 10.1177/0271678X211072759. Epub 2022 Feb 14. J Cereb Blood Flow Metab. 2022. PMID: 35156427 Free PMC article. No abstract available.
Abstract
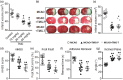
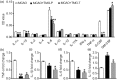
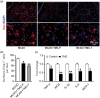
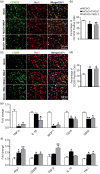
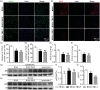
Inflammatory responses are accountable for secondary injury induced by acute ischemic stroke (AIS). Previous studies indicated that O-GlcNAc modification (O-GlcNAcylation) is involved in the pathology of AIS, and increase of O-GlcNAcylation by glucosamine attenuated the brain damage after ischemia/reperfusion. Inhibition of β-N-acetylglucosaminidase (OGA) with thiamet G (TMG) is an alternative option for accumulating O-GlcNAcylated proteins. In this study, we investigate the neuroprotective effect of TMG in a mouse model of experimental stroke. Our results indicate that TMG administration either before or after middle cerebral artery occlusion (MCAO) surgery dramatically reduced infarct volume compared with that in untreated controls. TMG treatment ameliorated the neurological deficits and improved clinical outcomes in neurobehavioral tests by modulating the expression of pro-inflammatory and anti-inflammatory cytokines. Additionally, TMG administration reduced the number of Iba1+ cells in MCAO mice, decreased expression of the M1 markers, and increased expression of the M2 markers in vivo. In vitro, M1 polarization of BV2 cells was inhibited by TMG treatment. Moreover, TMG decreased the expression of iNOS and COX2 mainly by suppressing NF-κB p65 signaling. These results suggest that TMG exerts a neuroprotective effect and could be useful as an anti-inflammatory agent for ischemic stroke therapy.
Keywords: Inflammation; macrophages; microglia; stroke; thiamet G.
Figures

References
-
- Emsley HC, Hopkins SJ. Acute ischaemic stroke and infection: recent and emerging concepts. Lancet Neurol 2008; 7: 341–353. - PubMed
-
- Li L, Qin JJ, Guo S, et al. Attenuation of cerebral ischemic injury in interferon regulatory factor 3 deficient rat. J Neurochem 2016; 136: 871–883. . - PubMed
-
- Ma Y, Wang J, Wang Y, et al. The biphasic function of microglia in ischemic stroke. Prog Neurobiol. Epub ahead of print 2 February 2016. pii: S0301-0082(15)30070-8. doi: 10.1016/j.pneurobio.2016.01.005. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

