Construction of a male sterility system for hybrid rice breeding and seed production using a nuclear male sterility gene
- PMID: 27864513
- PMCID: PMC5150371
- DOI: 10.1073/pnas.1613792113
Construction of a male sterility system for hybrid rice breeding and seed production using a nuclear male sterility gene
Erratum in
-
Correction for Chang et al., Construction of a male sterility system for hybrid rice breeding and seed production using a nuclear male sterility gene.Proc Natl Acad Sci U S A. 2017 Jan 3;114(1):E107. doi: 10.1073/pnas.1619974114. Epub 2016 Dec 27. Proc Natl Acad Sci U S A. 2017. PMID: 28028235 Free PMC article. No abstract available.
Abstract
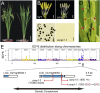
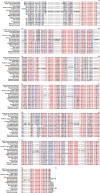
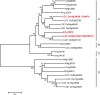
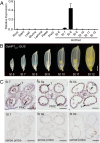
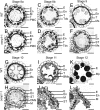
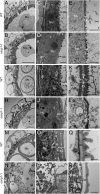
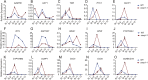
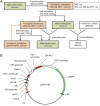
The breeding and large-scale adoption of hybrid seeds is an important achievement in agriculture. Rice hybrid seed production uses cytoplasmic male sterile lines or photoperiod/thermo-sensitive genic male sterile lines (PTGMS) as female parent. Cytoplasmic male sterile lines are propagated via cross-pollination by corresponding maintainer lines, whereas PTGMS lines are propagated via self-pollination under environmental conditions restoring male fertility. Despite huge successes, both systems have their intrinsic drawbacks. Here, we constructed a rice male sterility system using a nuclear gene named Oryza sativa No Pollen 1 (OsNP1). OsNP1 encodes a putative glucose-methanol-choline oxidoreductase regulating tapetum degeneration and pollen exine formation; it is specifically expressed in the tapetum and miscrospores. The osnp1 mutant plant displays normal vegetative growth but complete male sterility insensitive to environmental conditions. OsNP1 was coupled with an α-amylase gene to devitalize transgenic pollen and the red fluorescence protein (DsRed) gene to mark transgenic seed and transformed into the osnp1 mutant. Self-pollination of the transgenic plant carrying a single hemizygous transgene produced nontransgenic male sterile and transgenic fertile seeds in 1:1 ratio that can be sorted out based on the red fluorescence coded by DsRed Cross-pollination of the fertile transgenic plants to the nontransgenic male sterile plants propagated the male sterile seeds of high purity. The male sterile line was crossed with ∼1,200 individual rice germplasms available. Approximately 85% of the F1s outperformed their parents in per plant yield, and 10% out-yielded the best local cultivars, indicating that the technology is promising in hybrid rice breeding and production.
Keywords: OsNP1; breeding; hybrid rice; hybrid seed production; male sterility.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Chen L, Liu YG. Male sterility and fertility restoration in crops. Annu Rev Plant Biol. 2014;65:579–606. - PubMed
-
- Xu X, Zhang S, Liang K. Progress and discussion in breeding of indica rice CMS lines in China. Chin Agr Sci Bul. 2007;3:176–180.
-
- Chen L, Lei D, Tang W, Xiao Y. Thoughts and practice on some problems about research and application of two-line hybrid rice. Chin J Rice Sci. 2011;18(2):79–85.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

