Discovery of cofactor-specific, bactericidal Mycobacterium tuberculosis InhA inhibitors using DNA-encoded library technology
- PMID: 27864515
- PMCID: PMC5150407
- DOI: 10.1073/pnas.1610978113
Discovery of cofactor-specific, bactericidal Mycobacterium tuberculosis InhA inhibitors using DNA-encoded library technology
Abstract
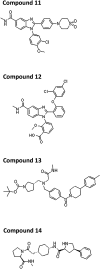
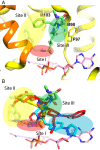
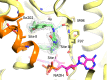
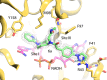
Millions of individuals are infected with and die from tuberculosis (TB) each year, and multidrug-resistant (MDR) strains of TB are increasingly prevalent. As such, there is an urgent need to identify novel drugs to treat TB infections. Current frontline therapies include the drug isoniazid, which inhibits the essential NADH-dependent enoyl-acyl-carrier protein (ACP) reductase, InhA. To inhibit InhA, isoniazid must be activated by the catalase-peroxidase KatG. Isoniazid resistance is linked primarily to mutations in the katG gene. Discovery of InhA inhibitors that do not require KatG activation is crucial to combat MDR TB. Multiple discovery efforts have been made against InhA in recent years. Until recently, despite achieving high potency against the enzyme, these efforts have been thwarted by lack of cellular activity. We describe here the use of DNA-encoded X-Chem (DEX) screening, combined with selection of appropriate physical properties, to identify multiple classes of InhA inhibitors with cell-based activity. The utilization of DEX screening allowed the interrogation of very large compound libraries (1011 unique small molecules) against multiple forms of the InhA enzyme in a multiplexed format. Comparison of the enriched library members across various screening conditions allowed the identification of cofactor-specific inhibitors of InhA that do not require activation by KatG, many of which had bactericidal activity in cell-based assays.
Keywords: DNA-encoded X-Chem technology; DNA-encoded libraries; InhA; Mycobacterium tuberculosis; multidrug resistance.
Conflict of interest statement
H.H.S., P.C., M.A.C., J.W.C., M.-A.G., S.H., A.D.K., K.M.K., E.A.S., and Y.Z. are employees of X-Chem Pharmaceuticals. DNA-encoded X-Chem technology (DEX) is a proprietary drug discovery platform discovered and developed by employees of X-Chem. A.D.F., G.D., E.R.S., J.B., P.M., and J.A.R. are employees of AstraZeneca.
Figures


References
-
- Banerjee A, et al. inhA, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosis. Science. 1994;263(5144):227–230. - PubMed
-
- Dessen A, Quémard A, Blanchard JS, Jacobs WR, Jr, Sacchettini JC. Crystal structure and function of the isoniazid target of Mycobacterium tuberculosis. Science. 1995;267(5204):1638–1641. - PubMed
-
- Rozwarski DA, Grant GA, Barton DHR, Jacobs WR, Jr, Sacchettini JC. Modification of the NADH of the isoniazid target (InhA) from Mycobacterium tuberculosis. Science. 1998;279(5347):98–102. - PubMed
-
- Chollet A, et al. Crystal structure of the enoyl-ACP reductase of Mycobacterium tuberculosis (InhA) in the apo-form and in complex with the active metabolite of isoniazid pre-formed by a biomimetic approach. J Struct Biol. 2015;190(3):328–337. - PubMed
-
- Schroeder EK, de Souza N, Santos DS, Blanchard JS, Basso LA. Drugs that inhibit mycolic acid biosynthesis in Mycobacterium tuberculosis. Curr Pharm Biotechnol. 2002;3(3):197–225. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

