Short Enantioselective Total Synthesis of Tatanan A and 3-epi-Tatanan A Using Assembly-Line Synthesis
- PMID: 27865037
- PMCID: PMC5215435
- DOI: 10.1002/anie.201609598
Short Enantioselective Total Synthesis of Tatanan A and 3-epi-Tatanan A Using Assembly-Line Synthesis
Abstract
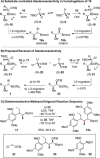
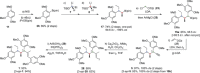
Short and highly stereoselective total syntheses of the sesquilignan natural product tatanan A and its C3 epimer are described. An assembly-line synthesis approach, using iterative lithiation-borylation reactions, was applied to install the three contiguous stereocenters with high enantio- and diastereoselectivity. One of the stereocenters was installed using a configurationally labile lithiated primary benzyl benzoate, resulting in high levels of substrate-controlled (undesired) diastereoselectivity. However, reversal of selectivity was achieved by using a novel diastereoselective Matteson homologation. Stereospecific alkynylation of a hindered secondary benzylic boronic ester enabled completion of the synthesis in a total of eight steps.
Keywords: Matteson homologation; alkynylation; lithiation-borylation; tatanan A; total synthesis.
© 2016 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Figures



Similar articles
-
Enantioselective installation of adjacent tertiary benzylic stereocentres using lithiation-borylation-protodeboronation methodology. Application to the synthesis of bifluranol and fluorohexestrol.Chem Sci. 2015 Jul 1;6(7):3718-3723. doi: 10.1039/c4sc03901g. Epub 2015 Apr 28. Chem Sci. 2015. PMID: 29218141 Free PMC article.
-
Lithiation-borylation methodology and its application in synthesis.Acc Chem Res. 2014 Oct 21;47(10):3174-83. doi: 10.1021/ar5002473. Epub 2014 Sep 29. Acc Chem Res. 2014. PMID: 25262745
-
Stereoselective Synthesis of Secondary and Tertiary Boronic Esters via Matteson Homologation.Org Lett. 2023 Sep 22;25(37):6835-6839. doi: 10.1021/acs.orglett.3c02360. Epub 2023 Sep 11. Org Lett. 2023. PMID: 37695257
-
Syntheses of Marine Natural Products via Matteson Homologations and Related Processes.Mar Drugs. 2025 Jan 2;23(1):20. doi: 10.3390/md23010020. Mar Drugs. 2025. PMID: 39852522 Free PMC article. Review.
-
Enantioselective total syntheses of several bioactive natural products based on the development of practical asymmetric catalysis.Chem Pharm Bull (Tokyo). 2004 Sep;52(9):1031-52. doi: 10.1248/cpb.52.1031. Chem Pharm Bull (Tokyo). 2004. PMID: 15340187 Review.
Cited by
-
Enantioselective γ-borylation of unsaturated amides and stereoretentive Suzuki-Miyaura cross-coupling.Chem Sci. 2017 Jun 1;8(6):4511-4516. doi: 10.1039/c7sc01093a. Epub 2017 May 3. Chem Sci. 2017. PMID: 28758006 Free PMC article.
-
A Straightforward Synthesis of Polyketides via Ester Dienolate Matteson Homologation.Chemistry. 2021 Jan 13;27(3):949-953. doi: 10.1002/chem.202004650. Epub 2020 Dec 15. Chemistry. 2021. PMID: 33089903 Free PMC article.
-
The Molecular Industrial Revolution: Automated Synthesis of Small Molecules.Angew Chem Int Ed Engl. 2018 Apr 9;57(16):4192-4214. doi: 10.1002/anie.201710482. Epub 2018 Mar 7. Angew Chem Int Ed Engl. 2018. PMID: 29513400 Free PMC article. Review.
-
Marine Puupehenone and Puupehedione: Synthesis and Future Perspectives.Mar Drugs. 2023 May 26;21(6):322. doi: 10.3390/md21060322. Mar Drugs. 2023. PMID: 37367647 Free PMC article. Review.
-
Synthesis of alkenyl boronates through stereoselective vinylene homologation of organoboronates.Nat Synth. 2024 Mar;3:337-346. doi: 10.1038/s44160-023-00461-w. Epub 2024 Jan 3. Nat Synth. 2024. PMID: 40821964 Free PMC article.
References
-
- None
-
- Oppolzer W., Moretti R., Bernardinelli G., Tetrahedron Lett. 1986, 27, 4713–4716;
-
- Hanessian S., Yang Y., Giroux S., Mascitti V., Ma J., Raeppel F., J. Am. Chem. Soc. 2003, 125, 13784–13792; - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

