Short Enantioselective Total Synthesis of Tatanan A and 3-epi-Tatanan A Using Assembly-Line Synthesis
- PMID: 27865037
- PMCID: PMC5215435
- DOI: 10.1002/anie.201609598
Short Enantioselective Total Synthesis of Tatanan A and 3-epi-Tatanan A Using Assembly-Line Synthesis
Abstract
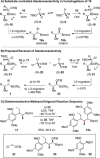
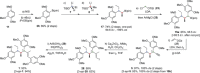
Short and highly stereoselective total syntheses of the sesquilignan natural product tatanan A and its C3 epimer are described. An assembly-line synthesis approach, using iterative lithiation-borylation reactions, was applied to install the three contiguous stereocenters with high enantio- and diastereoselectivity. One of the stereocenters was installed using a configurationally labile lithiated primary benzyl benzoate, resulting in high levels of substrate-controlled (undesired) diastereoselectivity. However, reversal of selectivity was achieved by using a novel diastereoselective Matteson homologation. Stereospecific alkynylation of a hindered secondary benzylic boronic ester enabled completion of the synthesis in a total of eight steps.
Keywords: Matteson homologation; alkynylation; lithiation-borylation; tatanan A; total synthesis.
© 2016 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Figures



References
-
- None
-
- Oppolzer W., Moretti R., Bernardinelli G., Tetrahedron Lett. 1986, 27, 4713–4716;
-
- Hanessian S., Yang Y., Giroux S., Mascitti V., Ma J., Raeppel F., J. Am. Chem. Soc. 2003, 125, 13784–13792; - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

