A quantitative proteomic screen of the Campylobacter jejuni flagellar-dependent secretome
- PMID: 27865792
- PMCID: PMC5223770
- DOI: 10.1016/j.jprot.2016.11.009
A quantitative proteomic screen of the Campylobacter jejuni flagellar-dependent secretome
Abstract
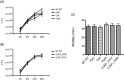
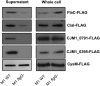
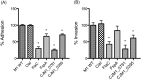
Campylobacter jejuni is the leading cause of bacterial gastroenteritis in the world. A number of factors are believed to contribute to the ability of C. jejuni to cause disease within the human host including the secretion of non-flagellar proteins via the flagellar type III secretion system (FT3SS). Here for the first time we have utilised quantitative proteomics using stable isotope labelling by amino acids in cell culture (SILAC), and label-free liquid chromatography-mass spectrometry (LC/MS), to compare supernatant samples from C. jejuni M1 wild type and flagella-deficient (flgG mutant) strains to identify putative novel proteins secreted via the FT3SS. Genes encoding proteins that were candidates for flagellar secretion, derived from the LC/MS and SILAC datasets, were deleted. Infection of human CACO-2 tissue culture cells using these mutants resulted in the identification of novel genes required for interactions with these cells. This work has shown for the first time that both CJM1_0791 and CJM1_0395 are dependent on the flagellum for their presence in supernatants from C. jejuni stains M1 and 81-176.
Biological significance: This study provides the most complete description of the Campylobac er jejuni secretome to date. SILAC and label-free proteomics comparing mutants with or without flagella have resulted in the identification of two C. jejuni proteins that are dependent on flagella for their export from the bacterial cell.
Keywords: Campylobacter jejuni; Flagella; Mass spec; SILAC; Secretome; Type III secretion system.
Copyright © 2016 The Authors. Published by Elsevier B.V. All rights reserved.
Figures




References
-
- Kirk M.D., Pires S.M., Black R.E., Caipo M., Crump J.A., Devleesschauwer B., Döpfer D., Fazil A., Fischer-Walker C.L., Hald T., Hall A.J., Keddy K.H., Lake R.J., Lanata C.F., Torgerson P.R., Havelaar A.H., Angulo F.J. World Health Organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: a data synthesis. PLoS Med. 2015;12 - PMC - PubMed
-
- Parkhill J., Wren B.W., Mungall K., Ketley J.M., Churcher C., Basham D., Chillingworth T., Davies R.M., Feltwell T., Holroyd S., Jagels K., Karlyshev A.V., Moule S., Pallen M.J., Penn C.W., Quail M.A., Rajandream M.A., Rutherford K.M., van Vliet A.H.M., Whitehead S., Barrell B.G. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature. 2000;403:665–668. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

