Neural Architecture of Hunger-Dependent Multisensory Decision Making in C. elegans
- PMID: 27866800
- PMCID: PMC5147516
- DOI: 10.1016/j.neuron.2016.10.030
Neural Architecture of Hunger-Dependent Multisensory Decision Making in C. elegans
Abstract
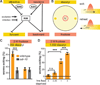
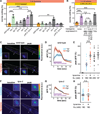
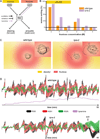
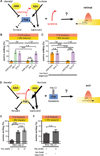
Little is known about how animals integrate multiple sensory inputs in natural environments to balance avoidance of danger with approach to things of value. Furthermore, the mechanistic link between internal physiological state and threat-reward decision making remains poorly understood. Here we confronted C. elegans worms with the decision whether to cross a hyperosmotic barrier presenting the threat of desiccation to reach a source of food odor. We identified a specific interneuron that controls this decision via top-down extrasynaptic aminergic potentiation of the primary osmosensory neurons to increase their sensitivity to the barrier. We also establish that food deprivation increases the worm's willingness to cross the dangerous barrier by suppressing this pathway. These studies reveal a potentially general neural circuit architecture for internal state control of threat-reward decision making.
Keywords: C. elegans; decision making; metabolism; multisensory integration; neural circuits; neuromodulation.
Copyright © 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
Author Information The authors declare no conflicts of interest.
Figures



References
-
- Alkema MJ, Hunter-Ensor M, Ringstad N, Horvitz HR. Tyramine Functions independently of octopamine in the Caenorhabditis elegans nervous system. Neuron. 2005;46:247–260. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

