Ethanol-seeking behavior is expressed directly through an extended amygdala to midbrain neural circuit
- PMID: 27866960
- PMCID: PMC5214453
- DOI: 10.1016/j.nlm.2016.11.013
Ethanol-seeking behavior is expressed directly through an extended amygdala to midbrain neural circuit
Abstract
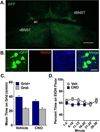
Abstinent alcohol-dependent individuals experience an enduring sensitivity to cue-induced craving and relapse to drinking. There is considerable evidence indicating that structures within the midbrain and extended amygdala are involved in this process. Individually, the ventral tegmental area (VTA) and the bed nucleus of the stria terminalis (BNST) have been shown to modulate cue-induced ethanol-seeking behavior. It is hypothesized that cue-induced seeking is communicated through a direct projection from the BNST to VTA. In the current experiments, an intersectional viral strategy was used in DBA/2J mice to selectively target and inhibit BNST projections to the VTA during a test of ethanol conditioned place preference (CPP). Inhibitory designer receptors exclusively activated by designer drugs (hM4Di DREADDs) were expressed in VTA-projecting BNST (BNST-VTA) cells by infusing a retrograde herpes-simplex virus encoding cre recombinase (HSV-Cre) into VTA and a cre-inducible adeno-associated virus encoding hM4Di (AAV-DIO-hM4Di) into BNST. Before testing the expression of preference, clozapine-N-oxide (CNO) was peripherally administered to activate hM4Di receptors and selectively inhibit these cells. Ethanol CPP expression was blocked by CNO-mediated inhibition of BNST-VTA cells. A follow-up study revealed this effect was specific to CNO activation of hM4Di as saline- and CNO-treated mice infused with a control vector (HSV-GFP) in place of HSV-Cre showed significant CPP. These findings establish a role for a direct BNST input to VTA in cue-induced ethanol-seeking behavior.
Keywords: BNST; CPP; DREADD; Ethanol; HSV; VTA.
Copyright © 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
The BNST balances alcohol's aversive and rewarding properties.Neuropsychopharmacology. 2019 Oct;44(11):1839-1840. doi: 10.1038/s41386-019-0374-z. Epub 2019 Apr 1. Neuropsychopharmacology. 2019. PMID: 30932025 Free PMC article. No abstract available.
References
-
- Adamantidis AR, Tsai H-C, Boutrel B, Zhang F, Stuber GD, Budygin EA, de Lecea L. Optogenetic interrogation of dopaminergic modulation of the multiple phases of reward-seeking behavior. The Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2011;31(30):10829–10835. http://doi.org/10.1523/JNEUROSCI.2246-11.2011. - DOI - PMC - PubMed
-
- Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(12):5163–5168. http://doi.org/10.1073/pnas.0700293104. - DOI - PMC - PubMed
-
- Beisker W, Dolbeare F, Gray JW. An improved immunocytochemical procedure for high-sensitivity detection of incorporated bromodeoxyuridine. Cytometry. 1987;8(2):235–239. http://doi.org/10.1002/cyto.990080218. - DOI - PubMed
-
- Boender AJ, de Jong JW, Boekhoudt L, Luijendijk MCM, van der Plasse G, Adan RAH. Combined use of the canine adenovirus-2 and DREADD-technology to activate specific neural pathways in vivo. PLoS ONE. 2014;9(4):e95392. http://doi.org/10.1371/journal.pone.0095392. - DOI - PMC - PubMed
-
- Buffalari DM, See RE. Inactivation of the bed nucleus of the stria terminalis in an animal model of relapse: effects on conditioned cue-induced reinstatement and its enhancement by yohimbine. Psychopharmacology. 2011;213(1):19–27. http://doi.org/10.1007/s00213-010-2008-3. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

