Clinical vascular imaging in the brain at 7T
- PMID: 27867089
- PMCID: PMC5862656
- DOI: 10.1016/j.neuroimage.2016.11.044
Clinical vascular imaging in the brain at 7T
Abstract
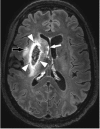
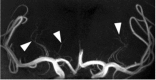
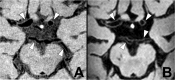
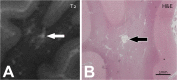
Stroke and related cerebrovascular diseases are a major cause of mortality and disability. Even at standard-field-strengths (1.5T), MRI is by far the most sensitive imaging technique to detect acute brain infarctions and to characterize incidental cerebrovascular lesions, such as white matter hyperintensities, lacunes and microbleeds. Arterial time-of-flight (TOF) MR angiography (MRA) can depict luminal narrowing or occlusion of the major brain feeding arteries, and this without the need for contrast administration. Compared to 1.5T MRA, the use of high-field strength (3T) and even more so ultra-high-field strengths (7T), enables the visualization of the lumen of much smaller intracranial vessels, while adding a contrast agent to TOF MRA at 7T may enable the visualization of even more distal arteries in addition to veins and venules. Moreover, with 3T and 7T, the arterial vessel walls beyond the circle of Willis become visible with high-resolution vessel wall imaging. In addition, with 7T MRI, the brain parenchyma can now be visualized on a submillimeter scale. As a result, high-resolution imaging studies of the brain and its blood supply at 7T have generated new concepts of different cerebrovascular diseases. In the current article, we will discuss emerging clinical applications and future directions of vascular imaging in the brain at 7T MRI.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Emerging Use of Ultra-High-Field 7T MRI in the Study of Intracranial Vascularity: State of the Field and Future Directions.AJNR Am J Neuroradiol. 2020 Jan;41(1):2-9. doi: 10.3174/ajnr.A6344. Epub 2019 Dec 26. AJNR Am J Neuroradiol. 2020. PMID: 31879330 Free PMC article. Review.
-
Advances in MR angiography with 7T MRI: From microvascular imaging to functional angiography.Neuroimage. 2018 Mar;168:269-278. doi: 10.1016/j.neuroimage.2017.01.019. Epub 2017 Jan 13. Neuroimage. 2018. PMID: 28089903 Review.
-
High-resolution postcontrast time-of-flight MR angiography of intracranial perforators at 7.0 Tesla.PLoS One. 2015 Mar 16;10(3):e0121051. doi: 10.1371/journal.pone.0121051. eCollection 2015. PLoS One. 2015. PMID: 25774881 Free PMC article.
-
Visualization of the lenticulostriate arteries at 3T using black-blood T1-weighted intracranial vessel wall imaging: comparison with 7T TOF-MRA.Eur Radiol. 2019 Mar;29(3):1452-1459. doi: 10.1007/s00330-018-5701-y. Epub 2018 Aug 27. Eur Radiol. 2019. PMID: 30151642
-
Cerebral lesions on 7 tesla MRI in patients with sickle cell anemia.Cerebrovasc Dis. 2015;39(3-4):181-9. doi: 10.1159/000373917. Epub 2015 Mar 5. Cerebrovasc Dis. 2015. PMID: 25765995
Cited by
-
Cerebral Small Vessel Disease in Population-Based Research: What are We Looking at - and What not?Aging Dis. 2024 Aug 1;15(4):1438-1446. doi: 10.14336/AD.2023.0323. Aging Dis. 2024. PMID: 37450929 Free PMC article.
-
Neuroimaging of Small Vessel Disease in Late-Life Depression.Adv Exp Med Biol. 2019;1192:95-115. doi: 10.1007/978-981-32-9721-0_5. Adv Exp Med Biol. 2019. PMID: 31705491 Free PMC article.
-
Imaging of intracranial atherosclerotic plaques using 3.0 T and 7.0 T magnetic resonance imaging-current trends and future perspectives.Cardiovasc Diagn Ther. 2020 Aug;10(4):994-1004. doi: 10.21037/cdt.2020.02.03. Cardiovasc Diagn Ther. 2020. PMID: 32968656 Free PMC article. Review.
-
Assessment of Heating on Titanium Alloy Cerebral Aneurysm Clips during 7T MRI.AJNR Am J Neuroradiol. 2022 Jul;43(7):972-977. doi: 10.3174/ajnr.A7561. Epub 2022 Jun 23. AJNR Am J Neuroradiol. 2022. PMID: 35738672 Free PMC article.
-
Advanced neuroimaging techniques for evaluating pediatric epilepsy.Clin Exp Pediatr. 2020 Mar;63(3):88-95. doi: 10.3345/kjp.2019.00871. Epub 2020 Feb 6. Clin Exp Pediatr. 2020. PMID: 32024331 Free PMC article.
References
-
- Aoki S., Shirouzu I., Sasaki Y. Enhancement of the intracranial arterial wall at MR imaging: relationship to cerebral atherosclerosis. Radiology. 1995;194:477–481. - PubMed
-
- Arai D., Satow T., Komuro T. Evaluation of the arterial wall in vertebrobasilar artery dissection using high-resolution magnetic resonance vessel wall imaging. J. Stroke Cerebrovasc. Dis. 2016 - PubMed
-
- Bernick C., Kuller L., Dulberg C. Silent MRI infarcts and the risk of future stroke: the cardiovascular health study. Neurology. 2001;57:1222–1229. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

