Ammonia promotes endothelial cell survival via the heme oxygenase-1-mediated release of carbon monoxide
- PMID: 27867098
- PMCID: PMC5209302
- DOI: 10.1016/j.freeradbiomed.2016.11.029
Ammonia promotes endothelial cell survival via the heme oxygenase-1-mediated release of carbon monoxide
Abstract
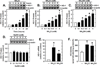
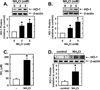
Although endothelial cells produce substantial quantities of ammonia during cell metabolism, the physiologic role of this gas in these cells is not known. In this study, we investigated if ammonia regulates the expression of heme oxygenase-1 (HO-1), and if this enzyme influences the biological actions of ammonia on endothelial cells. Exogenously administered ammonia, given as ammonium chloride or ammonium hydroxide, or endogenously generated ammonia stimulated HO-1 protein expression in cultured human and murine endothelial cells. Dietary supplementation of ammonia also induced HO-1 protein expression in murine arteries. The increase in HO-1 protein by ammonia in endothelial cells was first detected 4h after ammonia exposure and was associated with the induction of HO-1 mRNA, enhanced production of reactive oxygen species (ROS), and increased expression and activity of NF-E2-related factor-2 (Nrf2). Ammonia also activated the HO-1 promoter and this was blocked by mutating the antioxidant responsive element or by overexpressing dominant-negative Nrf2. The induction of HO-1 expression by ammonia was dependent on ROS formation and prevented by N-acetylcysteine or rotenone. Finally, prior treatment of endothelial cells with ammonia inhibited tumor necrosis factor-α-stimulated cell death. However, silencing HO-1 expression abrogated the protective action of ammonia and this was reversed by the administration of carbon monoxide but not bilirubin or iron. In conclusion, this study demonstrates that ammonia stimulates the expression of HO-1 in endothelial cells via the ROS-Nrf2 pathway, and that the induction of HO-1 contributes to the cytoprotective action of ammonia by generating carbon monoxide. Moreover, it identifies ammonia as a potentially important signaling gas in the vasculature that promotes endothelial cell survival.
Keywords: Ammonia; Carbon monoxide; Cytoprotection; Endothelial cells; Heme oxygenase-1.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures

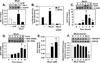


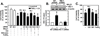
Similar articles
-
Heme oxygenase-1-derived bilirubin counteracts HIV protease inhibitor-mediated endothelial cell dysfunction.Free Radic Biol Med. 2016 May;94:218-29. doi: 10.1016/j.freeradbiomed.2016.03.003. Epub 2016 Mar 8. Free Radic Biol Med. 2016. PMID: 26968795 Free PMC article.
-
Hypochlorous acid-induced heme oxygenase-1 gene expression promotes human endothelial cell survival.Am J Physiol Cell Physiol. 2009 Oct;297(4):C907-15. doi: 10.1152/ajpcell.00536.2008. Epub 2009 Jul 22. Am J Physiol Cell Physiol. 2009. PMID: 19625608 Free PMC article.
-
Physiological cyclic strain promotes endothelial cell survival via the induction of heme oxygenase-1.Am J Physiol Heart Circ Physiol. 2013 Jun 15;304(12):H1634-43. doi: 10.1152/ajpheart.00872.2012. Epub 2013 Apr 19. Am J Physiol Heart Circ Physiol. 2013. PMID: 23604711 Free PMC article.
-
Compound C stimulates heme oxygenase-1 gene expression via the Nrf2-ARE pathway to preserve human endothelial cell survival.Biochem Pharmacol. 2011 Aug 15;82(4):371-9. doi: 10.1016/j.bcp.2011.05.016. Epub 2011 May 24. Biochem Pharmacol. 2011. PMID: 21635873 Free PMC article.
-
Carbon monoxide, reactive oxygen signaling, and oxidative stress.Free Radic Biol Med. 2008 Sep 1;45(5):562-9. doi: 10.1016/j.freeradbiomed.2008.05.013. Epub 2008 May 28. Free Radic Biol Med. 2008. PMID: 18549826 Free PMC article. Review.
Cited by
-
Heme Oxygenase 1: A Defensive Mediator in Kidney Diseases.Int J Mol Sci. 2021 Feb 18;22(4):2009. doi: 10.3390/ijms22042009. Int J Mol Sci. 2021. PMID: 33670516 Free PMC article. Review.
-
Apoptosis in resistance arteries induced by hydrogen peroxide: greater resilience of endothelium versus smooth muscle.Am J Physiol Heart Circ Physiol. 2021 Apr 1;320(4):H1625-H1633. doi: 10.1152/ajpheart.00956.2020. Epub 2021 Feb 19. Am J Physiol Heart Circ Physiol. 2021. PMID: 33606587 Free PMC article. Review.
-
miR-183-5p alleviates early injury after intracerebral hemorrhage by inhibiting heme oxygenase-1 expression.Aging (Albany NY). 2020 Jun 29;12(13):12869-12895. doi: 10.18632/aging.103343. Epub 2020 Jun 29. Aging (Albany NY). 2020. PMID: 32602850 Free PMC article.
-
Toxoplasma gondii-induced host's high secretion of 25-hydroxycholesterol for immunoprotection.Parasit Vectors. 2025 Aug 4;18(1):333. doi: 10.1186/s13071-025-06890-0. Parasit Vectors. 2025. PMID: 40760662 Free PMC article.
-
Recent advances on endogenous gasotransmitters in inflammatory dermatological disorders.J Adv Res. 2021 Sep 1;38:261-274. doi: 10.1016/j.jare.2021.08.012. eCollection 2022 May. J Adv Res. 2021. PMID: 35572410 Free PMC article. Review.
References
-
- Adeva MM, Sauto G, Blanco N, Donapetry C. Ammonium metabolism in humans. Metab Clin Exp. 2012;61:1495–1511. - PubMed
-
- Kopstein J, Wrong OM. The origin and fate of salivary urea and ammonium in man. Clin Sci Mol Med. 1977;52:9–17. - PubMed
-
- Meijer AJ, Lamers WH, Chamuleau RAFM. Nitrogen metabolism and ornithine cycle function. Physiol Rev. 1990;70:701–748. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

