Candida albicans cell-type switching and functional plasticity in the mammalian host
- PMID: 27867199
- PMCID: PMC5957277
- DOI: 10.1038/nrmicro.2016.157
Candida albicans cell-type switching and functional plasticity in the mammalian host
Abstract
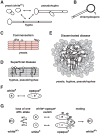
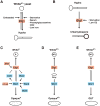
Candida albicans is a ubiquitous commensal of the mammalian microbiome and the most prevalent fungal pathogen of humans. A cell-type transition between yeast and hyphal morphologies in C. albicans was thought to underlie much of the variation in virulence observed in different host tissues. However, novel yeast-like cell morphotypes, including opaque(a/α), grey and gastrointestinally induced transition (GUT) cell types, were recently reported that exhibit marked differences in vitro and in animal models of commensalism and disease. In this Review, we explore the characteristics of the classic cell types - yeast, hyphae, pseudohyphae and chlamydospores - as well as the newly identified yeast-like morphotypes. We highlight emerging knowledge about the associations of these different morphotypes with different host niches and virulence potential, as well as the environmental cues and signalling pathways that are involved in the morphological transitions.
Conflict of interest statement
There is
Figures

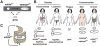
References
-
- Oyeka CA, Ugwu LO. Fungal flora of human toe webs. Mycoses. 2002;45:488–491. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

