Eucalyptals D and E, new cytotoxic phloroglucinols from the fruits of Eucalyptus globulus and assignment of absolute configuration
- PMID: 27867229
- PMCID: PMC5114019
- DOI: 10.1016/j.tetlet.2012.03.073
Eucalyptals D and E, new cytotoxic phloroglucinols from the fruits of Eucalyptus globulus and assignment of absolute configuration
Abstract
Two new phloroglucinols, named eucalyptals D (1) and E (2), along with a related known compound (euglobal-In-3, 3) were isolated from the fruits of Eucalyptus globulus. Their structures were established on the basis of extensive spectroscopic studies, revealing that they share a common 3,5-diformyl-isopentyl phloroglucinol unit, but each is instead coupled to a different sesquiterpenoid skeleton (aromadendrene in 1, cadinene in 2, and a spirosesquiterpene in 3). Compound 1 possessed an unusual seven-membered D ring with an ether bridge between C-2 of the aromadendrene moiety and C-2' of the aromatic unit. The absolute configuration of the isolates was defined by the comparison of experimental and calculated electronic circular dichroism (ECD) spectra. Compounds 1-3 exhibited significant in vitro cytotoxicities against a few human cancer cell lines (Huh-7, Jurkat, BGC-823, and KE-97) using the CellTiter-Glo™ luminescent cell viability assay method.
Keywords: Cytotoxicities; Eucalyptals; Eucalyptus globulus; Myrtaceae; Phloroglucinols.
Figures

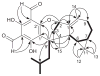
 ) and HMBC (H→C) correlations of 1.
) and HMBC (H→C) correlations of 1.
 H) correlations of 1 and 2.
H) correlations of 1 and 2.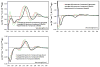

Similar articles
-
Eucalyptals A-C with a new skeleton isolated from Eucalyptus globulus.Org Lett. 2007 Dec 20;9(26):5549-52. doi: 10.1021/ol7025075. Epub 2007 Nov 17. Org Lett. 2007. PMID: 18020353
-
Formyl-phloroglucinol meroterpenoids with Nrf2 inhibitory activity from Eucalyptus globulus leaves.Phytochemistry. 2025 Jul;235:114457. doi: 10.1016/j.phytochem.2025.114457. Epub 2025 Feb 25. Phytochemistry. 2025. PMID: 40015583
-
Phloroglucinol Derivatives from the Fruits of Eucalyptus globulus and Their Cytotoxic Activities.Chem Biodivers. 2018 Jun;15(6):e1800052. doi: 10.1002/cbdv.201800052. Epub 2018 May 21. Chem Biodivers. 2018. PMID: 29692000
-
Eucalypglobulusals A-J, Formyl-Phloroglucinol-Terpene Meroterpenoids from Eucalyptus globulus Fruits.J Nat Prod. 2018 Dec 28;81(12):2638-2646. doi: 10.1021/acs.jnatprod.8b00430. Epub 2018 Dec 13. J Nat Prod. 2018. PMID: 30543429
-
Structural Diversity of Complex Phloroglucinol Derivatives from Eucalyptus Species.Chem Biodivers. 2022 Jun;19(6):e202200025. doi: 10.1002/cbdv.202200025. Epub 2022 May 27. Chem Biodivers. 2022. PMID: 35621714 Review.
Cited by
-
Configurational assignments of conformationally restricted bis-monoterpene hydroquinones: utility in exploration of endangered plants.Biochim Biophys Acta. 2013 Aug;1830(8):4229-34. doi: 10.1016/j.bbagen.2013.04.029. Epub 2013 Apr 26. Biochim Biophys Acta. 2013. PMID: 23628705 Free PMC article.
-
Acantholactone, a new manzamine related alkaloid with an unprecedented δ-lactone and ε-lactam ring system.Tetrahedron Lett. 2012 Nov 1;53(47):6329-6331. doi: 10.1016/j.tetlet.2012.08.140. Epub 2012 Sep 7. Tetrahedron Lett. 2012. PMID: 23526162 Free PMC article.
-
New Formyl Phloroglucinol Meroterpenoids from the Leaves of Eucalyptus robusta.Sci Rep. 2016 Dec 22;6:39815. doi: 10.1038/srep39815. Sci Rep. 2016. PMID: 28004790 Free PMC article.
References
-
- Eyles A, Davies NW, Mohammed C. J Chem Ecol. 2003;29:881–898. - PubMed
-
- Eschler BM, Pass DM, Willis R, Foley WJ. Biochem Syst Ecol. 2000;28:813–824. - PubMed
-
- Bharate SB, Singh IP. Bioorg Med Chem Lett. 2011;21:4310–4315. - PubMed
-
- Kawabata T, Hasegawa T, Nojiri Y, Uchida C, Tsubata T, Kato H, Takano F, Ohta T. Heterocycles. 2011;83:631–636.
-
- Editorial Commission of China Flora. Flora of China. Vol. 53. Science Press; Beijing: 1984. p. 28.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
