Bone muscle crosstalk targets muscle regeneration pathway regulated by core circadian transcriptional repressors DEC1 and DEC2
- PMID: 27867498
- PMCID: PMC5111231
- DOI: 10.1038/bonekey.2016.80
Bone muscle crosstalk targets muscle regeneration pathway regulated by core circadian transcriptional repressors DEC1 and DEC2
Abstract
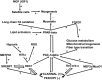
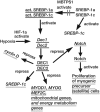
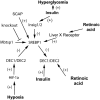
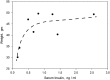
Deletion of proprotein convertase Mbtps1 in bone osteocytes leads to a significant postnatal increase in skeletal muscle size and contractile function, while causing only a 25% increase in stiffness in long bones. Concerns about leakiness in skeletal muscle were discounted since Cre recombinase expression does not account for our findings, and, Mbtps1 protein and mRNA is not deleted. Interestingly, the response of normal skeletal muscle to exercise and the regenerative response of skeletal muscle to the deletion of Mbtps1 in bone share some key regulatory features including a preference for slow twitch muscle fibers. In addition, transcriptional regulators PPAR, PGC-1α, LXR, and repressors DEC1 and DEC2 all occupy central positions within these two pathways. We hypothesize that the age-dependent muscle phenotype in Dmp1-Cre Mbtps1 cKO mice is due to bone→muscle crosstalk. Many of the myogenic genes altered in this larger and functionally improved muscle are regulated by circadian core transcriptional repressors DEC1 and DEC2, and furthermore, display a temporal coordination with Dec1 and Dec2 expression consistent with a regulatory co-dependency. These considerations lead us to propose that Dmp1-Cre Mbtps1 cKO osteocytes activate myogenesis by increased release of an activator of muscle PPAR-gamma, for example, PGE2 or sphingosine-1-P, or, by diminished release of an inhibitor of LXR, for example, long-chain polyunsaturated fatty acids. We hope that further investigation of these interacting pathways in the Dmp1-Cre Mbtps1 cKO model will lead to clinically translatable findings applicable to age-related sarcopenia and other muscle wasting syndromes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Deletion of Mbtps1 (Pcsk8, S1p, Ski-1) Gene in Osteocytes Stimulates Soleus Muscle Regeneration and Increased Size and Contractile Force with Age.J Biol Chem. 2016 Feb 26;291(9):4308-22. doi: 10.1074/jbc.M115.686626. Epub 2015 Dec 30. J Biol Chem. 2016. PMID: 26719336 Free PMC article.
-
Tissue-specific disruption of rhythmic expression of Dec1 and Dec2 in clock mutant mice.J Biol Rhythms. 2005 Oct;20(5):404-18. doi: 10.1177/0748730405280195. J Biol Rhythms. 2005. PMID: 16267380
-
DEC1 and DEC2 Crosstalk between Circadian Rhythm and Tumor Progression.J Cancer. 2016 Jan 1;7(2):153-9. doi: 10.7150/jca.13748. eCollection 2016. J Cancer. 2016. PMID: 26819638 Free PMC article. Review.
-
Regulation of basic helix-loop-helix transcription factors Dec1 and Dec2 by RORα and their roles in adipogenesis.Genes Cells. 2012 Feb;17(2):109-21. doi: 10.1111/j.1365-2443.2011.01574.x. Genes Cells. 2012. PMID: 22244086
-
Mechanisms involved in bone resorption regulated by vitamin D.J Steroid Biochem Mol Biol. 2018 Mar;177:70-76. doi: 10.1016/j.jsbmb.2017.11.005. Epub 2017 Nov 14. J Steroid Biochem Mol Biol. 2018. PMID: 29146302 Review.
Cited by
-
Muscle-Bone Crosstalk: Emerging Opportunities for Novel Therapeutic Approaches to Treat Musculoskeletal Pathologies.Biomedicines. 2017 Oct 24;5(4):62. doi: 10.3390/biomedicines5040062. Biomedicines. 2017. PMID: 29064421 Free PMC article. Review.
-
Key concepts in muscle regeneration: muscle "cellular ecology" integrates a gestalt of cellular cross-talk, motility, and activity to remodel structure and restore function.Eur J Appl Physiol. 2022 Feb;122(2):273-300. doi: 10.1007/s00421-021-04865-4. Epub 2021 Dec 20. Eur J Appl Physiol. 2022. PMID: 34928395 Free PMC article. Review.
-
Effects of ursolic acid on muscle mass and bone microstructure in rats with casting-induced muscle atrophy.J Exerc Nutrition Biochem. 2019 Sep 30;23(3):45-49. doi: 10.20463/jenb.2019.0022. J Exerc Nutrition Biochem. 2019. PMID: 31743975 Free PMC article. English.
-
Non-circadian aspects of BHLHE40 cellular function in cancer.Genes Cancer. 2020;11(1-2):1-19. doi: 10.18632/genesandcancer.201. Genes Cancer. 2020. PMID: 32577154 Free PMC article.
-
Overexpression of BHLHE41, correlated with DNA hypomethylation in 3'UTR region, promotes the growth of human clear cell renal cell carcinoma.Oncol Rep. 2019 Apr;41(4):2137-2147. doi: 10.3892/or.2019.7004. Epub 2019 Feb 7. Oncol Rep. 2019. PMID: 30816499 Free PMC article.
References
-
- Yang N, Garton F, North K. alpha-actinin-3 and performance. Med Sport Sci 2009; 54: 88–101. - PubMed
-
- Weiss A, Schiaffino S, Leinwand LA. Comparative sequence analysis of the complete human sarcomeric myosin heavy chain family: implications for functional diversity. J Mol Biol 1999; 290: 61–75. - PubMed
-
- Mathes AL, Lafyatis R. Role for Toll-like receptor 3 in muscle regeneration after cardiotoxin injury. Muscle Nerve 2011; 43: 733–740. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
