Shrimp Protein Hydrolysate Modulates the Timing of Proinflammatory Macrophages in Bupivacaine-Injured Skeletal Muscles in Rats
- PMID: 27868064
- PMCID: PMC5102708
- DOI: 10.1155/2016/5214561
Shrimp Protein Hydrolysate Modulates the Timing of Proinflammatory Macrophages in Bupivacaine-Injured Skeletal Muscles in Rats
Abstract
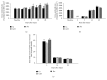
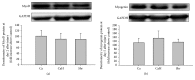
This study was designed to determine whether marine-derived proteins other than cod could have beneficial effects on inflammation following muscle injury. Macrophage and neutrophil densities were measured from bupivacaine-injured tibialis anterior muscle of rats fed isoenergetic diets containing either shrimp hydrolysate (Shr), casein hydrolysate (CaH), or whole casein (Ca). In this study, Shr reduced ED1+-macrophages at day 2 (p = 0.013), day 5 (p = 0.006), and day 14 after injury (p = 0.038) compared with Ca, indicating faster resolution of inflammation in Shr. Except for day 2 after injury where Shr led to lower ED1+-macrophages compared with CaH (p = 0.006), both Shr and CaH responded similarly at days 5, 14, and 28 after injury. This findings suggest that beneficial effects of Shr on ED1+-cells might be related to generation of anti-inflammatory peptides through the hydrolysis process, in addition to its high content of anti-inflammatory amino acids. However, while increasing myofiber cross-sectional area in noninjured muscles compared with both Ca and CaH, Shr failed to have a positive effect in corresponding injured muscles. These data indicate that shrimp hydrolysate can facilitate resolution of inflammation after muscle injury mainly through modulating proinflammatory macrophage accumulation but have less effect on optimal recovery in terms of muscle mass and fiber size.
Figures




References
-
- Lapointe B. M., Frenette J., Côté C. H. Lengthening contraction-induced inflammation is linked to secondary damage but devoid of neutrophil invasion. Journal of Applied Physiology. 2002;92(5):1995–2004. - PubMed
-
- Dort J., Leblanc N., Maltais-Giguère J., Liaset B., Côté C. H., Jacques H. Beneficial effects of cod protein on inflammatory cell accumulation in rat skeletal muscle after injury are driven by its high levels of arginine, glycine, taurine and lysine. PLoS ONE. 2013;8(10, article e77274) doi: 10.1371/journal.pone.0077274. - DOI - PMC - PubMed
-
- Tidball J. G. Mechanisms of muscle injury, repair, and regeneration. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2029;1(4):2029–2062. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

