Tuning the electrical conductance of metalloporphyrin supramolecular wires
- PMID: 27869128
- PMCID: PMC5116753
- DOI: 10.1038/srep37352
Tuning the electrical conductance of metalloporphyrin supramolecular wires
Abstract
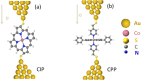
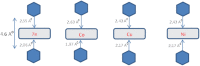
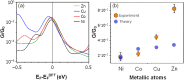
In contrast with conventional single-molecule junctions, in which the current flows parallel to the long axis or plane of a molecule, we investigate the transport properties of M(II)-5,15-diphenylporphyrin (M-DPP) single-molecule junctions (M=Co, Ni, Cu, or Zn divalent metal ions), in which the current flows perpendicular to the plane of the porphyrin. Novel STM-based conductance measurements combined with quantum transport calculations demonstrate that current-perpendicular-to-the-plane (CPP) junctions have three-orders-of-magnitude higher electrical conductances than their current-in-plane (CIP) counterparts, ranging from 2.10-2 G0 for Ni-DPP up to 8.10-2 G0 for Zn-DPP. The metal ion in the center of the DPP skeletons is strongly coordinated with the nitrogens of the pyridyl coated electrodes, with a binding energy that is sensitive to the choice of metal ion. We find that the binding energies of Zn-DPP and Co-DPP are significantly higher than those of Ni-DPP and Cu-DPP. Therefore when combined with its higher conductance, we identify Zn-DPP as the favoured candidate for high-conductance CPP single-molecule devices.
Figures

References
-
- Li Z., Smeu M., Ratner M. A. & Borguet E. Effect of anchoring groups on single molecule charge transport through porphyrins. The Journal of Physical Chemistry C 117, 14890–14898 (2013).
-
- Liu Z.-F. et al.. Control of Single-Molecule Junction Conductance of Porphyrins via a Transition-Metal Center. Nano letters 14, 5365–5370 (2014). - PubMed
-
- Suslick K. S., Rakow N. A., Kosal M. E. & Chou J.-H. The materials chemistry of porphyrins and metalloporphyrins. Journal of Porphyrins and Phthalocyanines 4, 407–413 (2000).
-
- Cárdenas-Jirón G. I. Aza nitrogens effect on the electronic properties of cobalt porphyrine and derivatives. Journal of the Chilean Chemical Society 49, 101–104 (2004).
-
- Auwärter W., Écija D., Klappenberger F. & Barth J. V. Porphyrins at interfaces. Nature chemistry 7, 105–120 (2015). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

