Assessing the Epidemic Potential of RNA and DNA Viruses
- PMID: 27869592
- PMCID: PMC5189130
- DOI: 10.3201/eid2212.160123
Assessing the Epidemic Potential of RNA and DNA Viruses
Abstract
Many new and emerging RNA and DNA viruses are zoonotic or have zoonotic origins in an animal reservoir that is usually mammalian and sometimes avian. Not all zoonotic viruses are transmissible (directly or by an arthropod vector) between human hosts. Virus genome sequence data provide the best evidence of transmission. Of human transmissible virus, 37 species have so far been restricted to self-limiting outbreaks. These viruses are priorities for surveillance because relatively minor changes in their epidemiologies can potentially lead to major changes in the threat they pose to public health. On the basis of comparisons across all recognized human viruses, we consider the characteristics of these priority viruses and assess the likelihood that they will further emerge in human populations. We also assess the likelihood that a virus that can infect humans but is not capable of transmission (directly or by a vector) between human hosts can acquire that capability.
Keywords: DNA viruses; RNA viruses; emerging viruses; epidemic potential; outbreaks; transmission; viruses; zoonoses.
Figures

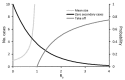
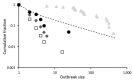

Comment in
-
Predicting pandemics.Lancet. 2016 Dec 17;388(10063):2960. doi: 10.1016/S0140-6736(16)32578-8. Lancet. 2016. PMID: 27998516 Free PMC article. No abstract available.
References
-
- King AM, Lefkowitz E, Adams MJ, Carstens EB. Virus taxonomy: ninth report of the International Committee on Taxonomy of Viruses. Amsterdam: Elsevier; 2012.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

