Highly Sensitive and Practical Detection of Plant Viruses via Electrical Impedance of Droplets on Textured Silicon-Based Devices
- PMID: 27869726
- PMCID: PMC5134605
- DOI: 10.3390/s16111946
Highly Sensitive and Practical Detection of Plant Viruses via Electrical Impedance of Droplets on Textured Silicon-Based Devices
Abstract
Early diagnosis of plant virus infections before the disease symptoms appearance may represent a significant benefit in limiting disease spread by a prompt application of appropriate containment steps. We propose a label-free procedure applied on a device structure where the electrical signal transduction is evaluated via impedance spectroscopy techniques. The device consists of a droplet suspension embedding two representative purified plant viruses i.e., Tomato mosaic virus and Turnip yellow mosaic virus, put in contact with a highly hydrophobic plasma textured silicon surface. Results show a high sensitivity of the system towards the virus particles with an interestingly low detection limit, from tens to hundreds of attomolar corresponding to pg/mL of sap, which refers, in the infection time-scale, to a concentration of virus particles in still-symptomless plants. Such a threshold limit, together with an envisaged engineering of an easily manageable device, compared to more sophisticated apparatuses, may contribute in simplifying the in-field plant virus diagnostics.
Keywords: EIS; TYMV; ToMV; droplet-based device; label-free detection; plant viruses; surface texturing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

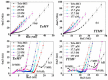

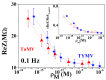
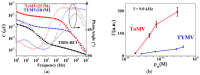
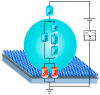

Similar articles
-
Label-free, high-throughput, electrical detection of cells in droplets.Analyst. 2013 Aug 21;138(16):4585-92. doi: 10.1039/c3an00569k. Epub 2013 Jun 10. Analyst. 2013. PMID: 23748871
-
Identification of a tobacco protein interacting with tomato mosaic virus coat protein and facilitating long-distance movement of virus.Arch Virol. 2005 Oct;150(10):1993-2008. doi: 10.1007/s00705-005-0554-5. Epub 2005 Jun 3. Arch Virol. 2005. PMID: 15931463
-
Nanotextured superhydrophobic electrodes enable detection of attomolar-scale DNA concentration within a droplet by non-faradaic impedance spectroscopy.Lab Chip. 2013 Nov 7;13(21):4248-56. doi: 10.1039/c3lc50517k. Lab Chip. 2013. PMID: 24056864 Free PMC article.
-
Factors involved in the systemic transport of plant RNA viruses: the emerging role of the nucleus.J Exp Bot. 2014 Apr;65(7):1689-97. doi: 10.1093/jxb/ert449. Epub 2014 Jan 13. J Exp Bot. 2014. PMID: 24420565 Review.
-
Toward a quarter century of pathogen-derived resistance and practical approaches to plant virus disease control.Adv Virus Res. 2009;75:161-83. doi: 10.1016/S0065-3527(09)07505-8. Epub 2010 Jan 13. Adv Virus Res. 2009. PMID: 20109666 Review.
Cited by
-
Getting Hold of the Tobamovirus Particle-Why and How? Purification Routes over Time and a New Customizable Approach.Viruses. 2024 May 30;16(6):884. doi: 10.3390/v16060884. Viruses. 2024. PMID: 38932176 Free PMC article. Review.
References
-
- Makkouk K.M., Hsu H.T., Kumari S.G. Detection of three plant viruses by dot-blot and tissue-blot immunoassays using chemiluminescent and chromogenic substrates. J. Phytopathol. 1993;139:97–102. doi: 10.1111/j.1439-0434.1993.tb01405.x. - DOI
-
- Fenby N.S., Scott N.W., Slater A., Elliott M.C. PCR and non-isotopic labeling techniques for plant virus detection. Cell. Mol. Biol. 1995;41:639–652. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

