Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system
- PMID: 27869805
- PMCID: PMC5562951
- DOI: 10.1038/nm.4233
Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system
Abstract
The enteric nervous system (ENS) of the gastrointestinal tract controls many diverse functions, including motility and epithelial permeability. Perturbations in ENS development or function are common, yet there is no human model for studying ENS-intestinal biology and disease. We used a tissue-engineering approach with embryonic and induced pluripotent stem cells (PSCs) to generate human intestinal tissue containing a functional ENS. We recapitulated normal intestinal ENS development by combining human-PSC-derived neural crest cells (NCCs) and developing human intestinal organoids (HIOs). NCCs recombined with HIOs in vitro migrated into the mesenchyme, differentiated into neurons and glial cells and showed neuronal activity, as measured by rhythmic waves of calcium transients. ENS-containing HIOs grown in vivo formed neuroglial structures similar to a myenteric and submucosal plexus, had functional interstitial cells of Cajal and had an electromechanical coupling that regulated waves of propagating contraction. Finally, we used this system to investigate the cellular and molecular basis for Hirschsprung's disease caused by a mutation in the gene PHOX2B. This is, to the best of our knowledge, the first demonstration of human-PSC-derived intestinal tissue with a functional ENS and how this system can be used to study motility disorders of the human gastrointestinal tract.
Figures
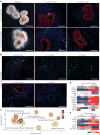



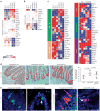
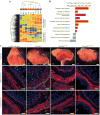
Comment in
-
Neurogastroenterology: Engineering human intestinal organoids with a functional ENS.Nat Rev Gastroenterol Hepatol. 2017 Jan;14(1):2. doi: 10.1038/nrgastro.2016.192. Epub 2016 Nov 30. Nat Rev Gastroenterol Hepatol. 2017. PMID: 27899814 No abstract available.
-
Created of Warm Blood and Nerves: Restoring an Enteric Nervous System in Organoids.Cell Stem Cell. 2017 Jan 5;20(1):5-7. doi: 10.1016/j.stem.2016.12.012. Cell Stem Cell. 2017. PMID: 28061353
References
-
- Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9:286–294. - PubMed
-
- Sasselli V, Pachnis V, Burns AJ. The enteric nervous system. Dev Biol. 2012;366:64–73. - PubMed
-
- Obermayr F, Hotta R, Enomoto H, Young HM. Development and developmental disorders of the enteric nervous system. Nat Rev Gastroenterol Hepatol. 2013;10:43–57. - PubMed
-
- Saffrey MJ. Cellular changes in the enteric nervous system during ageing. Dev Biol. 2013;382:344–355. - PubMed
-
- McKeown SJ, Stamp L, Hao MM, Young HM. Hirschsprung disease: a developmental disorder of the enteric nervous system. Wiley Interdiscip Rev Dev Biol. 2013;2:113–129. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

