Timing of Maternal Exposure and Foetal Sex Determine the Effects of Low-level Chemical Mixture Exposure on the Foetal Neuroendocrine System in Sheep
- PMID: 27870155
- PMCID: PMC5621486
- DOI: 10.1111/jne.12444
Timing of Maternal Exposure and Foetal Sex Determine the Effects of Low-level Chemical Mixture Exposure on the Foetal Neuroendocrine System in Sheep
Abstract
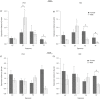
We have shown that continuous maternal exposure to the complex mixture of environmental chemicals (ECs) found in human biosolids (sewage sludge), disrupts mRNA expression of genes crucial for development and long-term regulation of hypothalamic-pituitary gonadal (HPG) function in sheep. The present study investigated whether exposure to ECs only during preconceptional period or only during pregnancy perturbed key regulatory genes within the hypothalamus and pituitary gland and whether these effects were different from chronic (life-long) exposure to biosolid ECs. The findings demonstrate that the timing and duration of maternal EC exposure influences the subsequent effects on the foetal neuroendocrine system in a sex-specific manner. Maternal exposure prior to conception, or during pregnancy only, altered the expression of key foetal neuroendocrine regulatory systems such as gonadotrophin-releasing hormone and kisspeptin to a greater extent than when maternal exposure was 'life-long'. Furthermore, hypothalamic gene expression was affected to a greater extent in males than in females and, following EC exposure, male foetuses expressed more 'female-like' mRNA levels for some key neuroendocrine genes. This is the first study to show that 'real-life' maternal exposure to low levels of a complex cocktail of chemicals prior to conception can subsequently affect the developing foetal neuroendocrine system. These findings demonstrate that the developing neuroendocrine system is sensitive to EC mixtures in a sex-dimorphic manner likely to predispose to reproductive dysfunction in later life.
Keywords: GnRH; endocrine disruptors; foetal; hypothalamus; kisspeptin; oestrogen receptor.
© 2016 The Authors. Journal of Neuroendocrinology published by John Wiley & Sons Ltd on behalf of British Society for Neuroendocrinology.
Figures
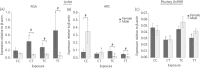


References
-
- Barker D, Eriksson J, Forsén T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol 2002; 31: 1235–1239. - PubMed
-
- Seckl JR. Prenatal glucocorticoids and long‐term programming. Eur J Endocrinol. 2004; 151(Suppl 3): U49–U62. - PubMed
-
- Grandjean P, Barouki R, Bellinger DC, Casteleyn L, Chadwick LH, Cordier S, Etzel RA, Gray KA, Ha EH, Junien C, Karagas M, Kawamoto T, Paige Lawrence B, Perera FP, Prins GS, Puga A, Rosenfeld CS, Sherr DH, Sly PD, Suk W, Sun Q, Toppari J, van den Hazel P, Walker CL, Heindel JJ. Life‐long implications of developmental exposure to environmental stressors: new perspectives. Endocrinology 2015; 156: 3408–3415. - PMC - PubMed
-
- Rogers JM. Tobacco and pregnancy: overview of exposures and effects. Birth Defects Res C Embryo Today 2008; 84: 1–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

