Understanding the broad influence of sex hormones and sex differences in the brain
- PMID: 27870427
- PMCID: PMC5120618
- DOI: 10.1002/jnr.23809
Understanding the broad influence of sex hormones and sex differences in the brain
Abstract
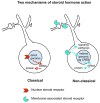
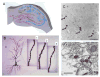
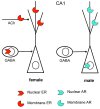
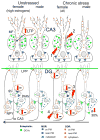
Sex hormones act throughout the entire brain of both males and females via both genomic and nongenomic receptors. Sex hormones can act through many cellular and molecular processes that alter structure and function of neural systems and influence behavior as well as providing neuroprotection. Within neurons, sex hormone receptors are found in nuclei and are also located near membranes, where they are associated with presynaptic terminals, mitochondria, spine apparatus, and postsynaptic densities. Sex hormone receptors also are found in glial cells. Hormonal regulation of a variety of signaling pathways as well as direct and indirect effects on gene expression induce spine synapses, up- or downregulate and alter the distribution of neurotransmitter receptors, and regulate neuropeptide expression and cholinergic and GABAergic activity as well as calcium sequestration and oxidative stress. Many neural and behavioral functions are affected, including mood, cognitive function, blood pressure regulation, motor coordination, pain, and opioid sensitivity. Subtle sex differences exist for many of these functions that are developmentally programmed by hormones and by not yet precisely defined genetic factors, including the mitochondrial genome. These sex differences and responses to sex hormones in brain regions, which influence functions not previously regarded as subject to such differences, indicate that we are entering a new era of our ability to understand and appreciate the diversity of gender-related behaviors and brain functions. © 2016 Wiley Periodicals, Inc.
Keywords: cardiovascular; cerebellum; estrogens; hippocampus; prefrontal cortex; stress.
© 2016 Wiley Periodicals, Inc.
Conflict of interest statement
The authors have no conflicts to declare.
Figures


References
-
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

