High Frequency and Poor Outcome of Philadelphia Chromosome-Like Acute Lymphoblastic Leukemia in Adults
- PMID: 27870571
- PMCID: PMC5455698
- DOI: 10.1200/JCO.2016.69.0073
High Frequency and Poor Outcome of Philadelphia Chromosome-Like Acute Lymphoblastic Leukemia in Adults
Abstract
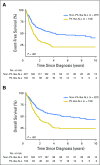
Purpose Philadelphia chromosome (Ph) -like acute lymphoblastic leukemia (ALL) is a high-risk subtype of childhood ALL characterized by kinase-activating alterations that are amenable to treatment with tyrosine kinase inhibitors. We sought to define the prevalence and genomic landscape of Ph-like ALL in adults and assess response to conventional chemotherapy. Patients and Methods The frequency of Ph-like ALL was assessed by gene expression profiling of 798 patients with B-cell ALL age 21 to 86 years. Event-free survival and overall survival were determined for Ph-like ALL versus non-Ph-like ALL patients. Detailed genomic analysis was performed on 180 of 194 patients with Ph-like ALL. Results Patients with Ph-like ALL accounted for more than 20% of adults with ALL, including 27.9% of young adults (age 21 to 39 years), 20.4% of adults (age 40 to 59 years), and 24.0% of older adults (age 60 to 86 years). Overall, patients with Ph-like ALL had an inferior 5-year event-free survival compared with patients with non-Ph-like ALL (22.5% [95% CI, 14.9% to 29.3%; n = 155] v 49.3% [95% CI, 42.8% to 56.2%; n = 247], respectively; P < .001). We identified kinase-activating alterations in 88% of patients with Ph-like ALL, including CRLF2 rearrangements (51%), ABL class fusions (9.8%), JAK2 or EPOR rearrangements (12.4%), other JAK-STAT sequence mutations (7.2%), other kinase alterations (4.1%), and Ras pathway mutations (3.6%). Eleven new kinase rearrangements were identified, including four involving new kinase or cytokine receptor genes and seven involving new partners for previously identified genes. Conclusion Ph-like ALL is a highly prevalent subtype of ALL in adults and is associated with poor outcome. The diverse range of kinase-activating alterations in Ph-like ALL has important therapeutic implications. Trials comparing the addition of tyrosine kinase inhibitors to conventional therapy are required to evaluate the clinical utility of these agents in the treatment of Ph-like ALL.
Figures

References
-
- Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in children. N Engl J Med. 2015;373:1541–1552. - PubMed
-
- Goldstone AH, Richards SM, Lazarus HM, et al. In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: Final results of the International ALL Trial (MRC UKALL XII/ECOG E2993) Blood. 2008;111:1827–1833. - PubMed
-
- Fielding AK, Richards SM, Chopra R, et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL): An MRC UKALL12/ECOG 2993 study. Blood. 2007;109:944–950. - PubMed
-
- Moorman AV, Ensor HM, Richards SM, et al. Prognostic effect of chromosomal abnormalities in childhood B-cell precursor acute lymphoblastic leukaemia: Results from the UK Medical Research Council ALL97/99 randomised trial. Lancet Oncol. 2010;11:429–438. - PubMed
-
- Moorman AV, Harrison CJ, Buck GA, et al. Karyotype is an independent prognostic factor in adult acute lymphoblastic leukemia (ALL): Analysis of cytogenetic data from patients treated on the Medical Research Council (MRC) UKALLXII/Eastern Cooperative Oncology Group (ECOG) 2993 trial. Blood. 2007;109:3189–3197. - PubMed
MeSH terms
Grants and funding
- U10 CA180861/CA/NCI NIH HHS/United States
- U10 CA013650/CA/NCI NIH HHS/United States
- U10 CA014958/CA/NCI NIH HHS/United States
- U24 CA196172/CA/NCI NIH HHS/United States
- U10 CA017145/CA/NCI NIH HHS/United States
- P30 CA021765/CA/NCI NIH HHS/United States
- U10 CA180790/CA/NCI NIH HHS/United States
- U01 CA157937/CA/NCI NIH HHS/United States
- HHSN261200800001C/RC/CCR NIH HHS/United States
- U10 CA021115/CA/NCI NIH HHS/United States
- UG1 CA189859/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- U10 CA180791/CA/NCI NIH HHS/United States
- U10 CA180850/CA/NCI NIH HHS/United States
- U24 CA196171/CA/NCI NIH HHS/United States
- P30 CA016058/CA/NCI NIH HHS/United States
- RC1 CA145707/CA/NCI NIH HHS/United States
- U10 CA180820/CA/NCI NIH HHS/United States
- U10 CA015488/CA/NCI NIH HHS/United States
- HHSN261200800001E/CA/NCI NIH HHS/United States
- U10 CA180827/CA/NCI NIH HHS/United States
- P50 GM115279/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

