Evaluation of BGJ398, a Fibroblast Growth Factor Receptor 1-3 Kinase Inhibitor, in Patients With Advanced Solid Tumors Harboring Genetic Alterations in Fibroblast Growth Factor Receptors: Results of a Global Phase I, Dose-Escalation and Dose-Expansion Study
- PMID: 27870574
- PMCID: PMC6865065
- DOI: 10.1200/JCO.2016.67.2048
Evaluation of BGJ398, a Fibroblast Growth Factor Receptor 1-3 Kinase Inhibitor, in Patients With Advanced Solid Tumors Harboring Genetic Alterations in Fibroblast Growth Factor Receptors: Results of a Global Phase I, Dose-Escalation and Dose-Expansion Study
Erratum in
Abstract
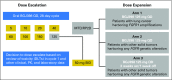
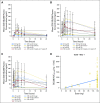
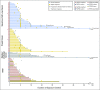
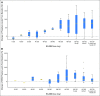
Purpose This two-part, first-in-human study was initiated in patients with advanced solid tumors harboring genetic alterations in fibroblast growth factor receptors (FGFRs) to determine the maximum tolerated dose (MTD), the recommended phase II dose (RP2D), and the schedule, safety, pharmacokinetics, pharmacodynamics, and antitumor activity of oral BGJ398, a selective FGFR1-3 tyrosine kinase inhibitor. Patients and Methods Adult patients were treated with escalating dosages of BGJ398 5 to 150 mg once daily or 50 mg twice daily continuously in 28-day cycles. During expansion at the MTD, patients with FGFR1-amplified squamous cell non-small-cell lung cancer (sqNSCLC; arm 1) or other solid tumors with FGFR genetic alterations (mutations/amplifications/fusions) received BGJ398 daily on a continuous schedule (arm 2), or on a 3-weeks-on/1-week-off schedule (arm 3). Results Data in 132 patients from the escalation and expansion arms are reported (May 15, 2015, cutoff). The MTD, 125 mg daily, was determined on the basis of dose-limiting toxicities in four patients (100 mg, grade 3 aminotransferase elevations [n = 1]; 125 mg, hyperphosphatemia [n = 1]; 150 mg, grade 1 corneal toxicity [n = 1] and grade 3 aminotransferase elevations [n = 1]). Common adverse events in patients treated at the MTD (n = 57) included hyperphosphatemia (82.5%), constipation (50.9%), decreased appetite (45.6%), and stomatitis (45.6%). A similar safety profile was observed using the 3-weeks-on/1-week-off schedule (RP2D). However, adverse event-related dose adjustments/interruptions were less frequent with the 3-weeks-on/1-week-off (50.0%) versus the continuous (73.7%) schedule. Antitumor activity (seven partial responses [six confirmed]) was demonstrated with BGJ398 doses ≥ 100 mg in patients with FGFR1-amplified sqNSCLC and FGFR3-mutant bladder/urothelial cancer. Conclusion BGJ398 at the MTD/RP2D had a tolerable and manageable safety profile and showed antitumor activity in several tumor types, including FGFR1-amplified sqNSCLC and FGFR3-mutant bladder/urothelial cancers.
Figures

Comment in
-
Genome-Driven Paradigm for the Development of Selective Fibroblast Growth Factor Receptor Inhibitors.J Clin Oncol. 2017 Jan 10;35(2):131-134. doi: 10.1200/JCO.2016.70.5061. Epub 2016 Dec 5. J Clin Oncol. 2017. PMID: 27918716 No abstract available.
References
-
- Turner N, Grose R. Fibroblast growth factor signalling: From development to cancer. Nat Rev Cancer. 2010;10:116–129. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

