Clonal fate mapping quantifies the number of haematopoietic stem cells that arise during development
- PMID: 27870830
- PMCID: PMC5535785
- DOI: 10.1038/ncb3444
Clonal fate mapping quantifies the number of haematopoietic stem cells that arise during development
Erratum in
-
Corrigendum: Clonal fate mapping quantifies the number of haematopoietic stem cells that arise during development.Nat Cell Biol. 2017 Jan 31;19(2):142. doi: 10.1038/ncb3462. Nat Cell Biol. 2017. PMID: 28139650 Free PMC article. No abstract available.
Abstract
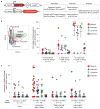
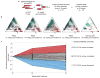
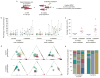
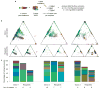
Haematopoietic stem cells (HSCs) arise in the developing aorta during embryogenesis. The number of HSC clones born has been estimated through transplantation, but experimental approaches to assess the absolute number of forming HSCs in a native setting have remained challenging. Here, we applied single-cell and clonal analysis of HSCs in zebrafish to quantify developing HSCs. Targeting creERT2 in developing cd41:eGFP+ HSCs enabled long-term assessment of their blood contribution. We also applied the Brainbow-based multicolour Zebrabow system with drl:creERT2 that is active in early haematopoiesis to induce heritable colour barcoding unique to each HSC and its progeny. Our findings reveal that approximately 21 HSC clones exist prior to HSC emergence and 30 clones are present during peak production from aortic endothelium. Our methods further reveal that stress haematopoiesis, including sublethal irradiation and transplantation, reduces clonal diversity. Our findings provide quantitative insights into the early clonal events that regulate haematopoietic development.
Conflict of interest statement
L.Z. is a founder and stock holder of Fate, Inc., and Scholar Rock.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

