Cytomegalovirus Reinfections Stimulate CD8 T-Memory Inflation
- PMID: 27870919
- PMCID: PMC5117776
- DOI: 10.1371/journal.pone.0167097
Cytomegalovirus Reinfections Stimulate CD8 T-Memory Inflation
Abstract
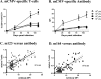
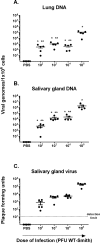
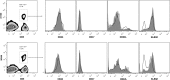
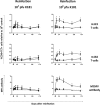
Cytomegalovirus (CMV) has been shown to induce large populations of CD8 T-effector memory cells that unlike central memory persist in large quantities following infection, a phenomenon commonly termed "memory inflation". Although murine models to date have shown very large and persistent CMV-specific T-cell expansions following infection, there is considerable variability in CMV-specific T-memory responses in humans. Historically such memory inflation in humans has been assumed a consequence of reactivation events during the life of the host. Because basic information about CMV infection/re-infection and reactivation in immune competent humans is not available, we used a murine model to test how primary infection, reinfection, and reactivation stimuli influence memory inflation. We show that low titer infections induce "partial" memory inflation of both mCMV specific CD8 T-cells and antibody. We show further that reinfection with different strains can boost partial memory inflation. Finally, we show preliminary results suggesting that a single strong reactivation stimulus does not stimulate memory inflation. Altogether, our results suggest that while high titer primary infections can induce memory inflation, reinfections during the life of a host may be more important than previously appreciated.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Reddehase MJ, Keil GM, Koszinowski UH. The cytolytic T lymphocyte response to the murine cytomegalovirus. II. Detection of virus replication stage-specific antigens by separate populations of in vivo active cytolytic T lymphocyte precursors. Eur J Immunol. 1984;14(1):56–61. 10.1002/eji.1830140111 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

